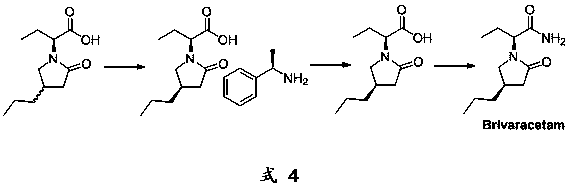
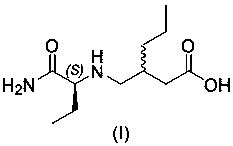
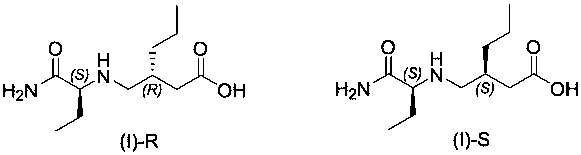Brivaracetam intermediate and preparation method thereof
An amino and compound technology, which is applied in the preparation of carboxylic acid amide optical isomers, carboxylate salts, and carboxylic acid amides, etc., can solve the problems of complicated operation, long synthesis route, and long synthesis scheme route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of 5-hydroxy-4-propylfuran-2(5H)-one (III)
[0023]
[0024] Add 125mL of n-heptane and 30mL of morpholine into a three-necked flask, stir at room temperature for 10min to mix evenly, and cool to 4 o Below C, 25.0 g of 50% glyoxylic acid aqueous solution was added dropwise. After dripping, the temperature is raised to 25~30 o C stirred the reaction for 2h, then at 40 o Below C, 30.5 g of n-valeraldehyde was slowly added, and the stirring reaction was continued for 18 h. After the reaction was completed, the temperature was lowered to 20 o C, slowly dropwise add concentrated hydrochloric acid 21.3g and stir.
[0025] Cool down to room temperature, let stand to separate the n-heptane phase. Add 100 mL of ethyl acetate to the water phase, slowly add solid sodium carbonate to adjust the pH to 4, separate the organic phase, extract it with ethyl acetate, wash the combined organic phase with saturated brine, dry over anhydrous sodium sulfate, an...
Embodiment 2
[0026] Embodiment 2: (2 S Preparation of )-2-(2-hydroxy-5-oxo-3-propyl-2,5-dihydro-1H-pyrrol-1-yl)butanamide (II)
[0027]
[0028] Mix 96.2 g of aminobutyramide hydrochloride in 1000 mL of isopropanol, and release it with ammonia gas until the pH value of the system is 9~10 and the pH value does not change. The salt was removed by filtration, and the filtrate was concentrated to 500 mL for use.
[0029] Add 98.4 g of 5-hydroxy-4-propylfuran-2(5H)-one (III) in batches to the above-mentioned 500 mL aminobutyramide solution, and control the temperature at 30~40 o C reaction more than 2h. After the reaction was completed, the salt was removed by filtration, and the filtrate was slowly cooled to 0-5 o C crystallization, suction filtration, rinsing with a small amount of ethyl acetate to give white solid (2S)-2-(2-hydroxy-5-oxo-3-propyl-2,5-dihydro-1H-pyrrole-1 -yl) butanamide (II) 139.2g, yield 88.8%. 1 H NMR (500 MHz, Chloroform- d ) δ 6.45 (s, 1H),5.89 (s, 1H), 5.85 (s,...
Embodiment 3
[0030] Example 3: Preparation of 3-((((S)-1-amino-1-oxobut-2-yl)amino)methyl)hexanoic acid (I)
[0031]
[0032] Add 15.0 g of (2S)-2-(2-hydroxy-5-oxo-3-propyl-2,5-dihydro-1H-pyrrol-1-yl)butanamide (II) into 150 mL of water, and then Add 1.5g of 5% Pd / C, stir evenly, and replace with nitrogen. Hydrogen was passed to 20 bar, and the reaction was stirred overnight at room temperature. After the reaction, the palladium carbon was filtered off, and the filtrate was spin-dried to obtain light yellow oil 3-((((S)-1-amino-1-oxobut-2-yl)amino)methyl)hexanoic acid (I) 16.3g, yield 93.7%. 1 H NMR (400 MHz, DMSO- d 6 ) δ 7.34 (s, 1H),7.01 (s, 1H), 2.96 – 2.79 (m, 1H), 2.56 (dd, J = 11.6, 4.9 Hz, 1H), 2.40 (d, J = 4.8 Hz, 1H), 2.29 (ddt, J= 28.6, 20.1, 10.3 Hz, 1H), 2.13 (ddd, J =21.1, 11.9, 5.2 Hz, 1H), 1.93 – 1.74 (m, 1H), 1.49 (tt, J = 13.6, 6.5 Hz,2H), 1.41 – 1.07 (m, 4H), 0.82 (dt, J = 29.2, 6.6 Hz, 6H). MS (ESI) m / z =231(M + +1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com