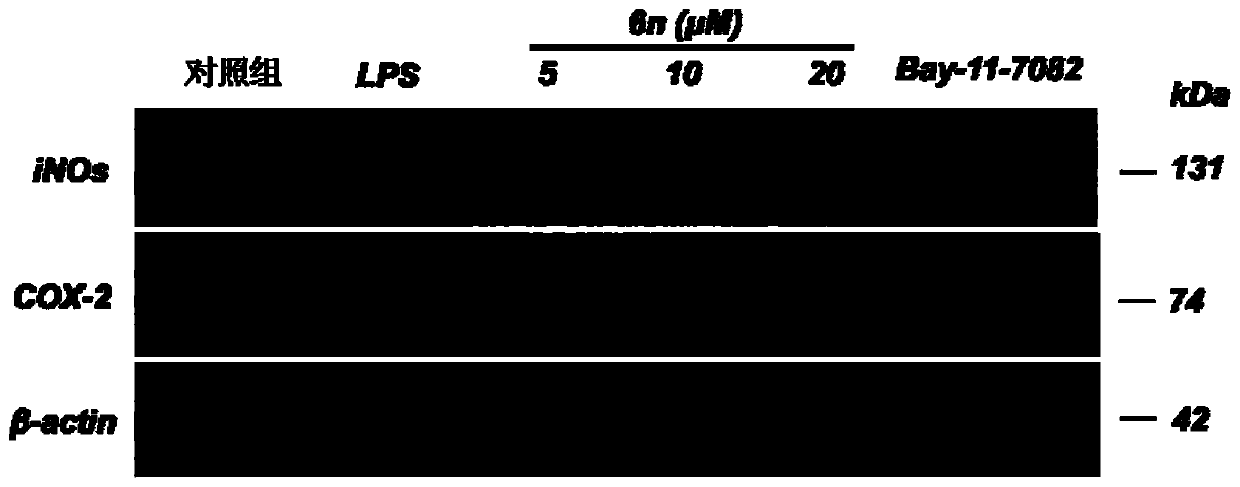
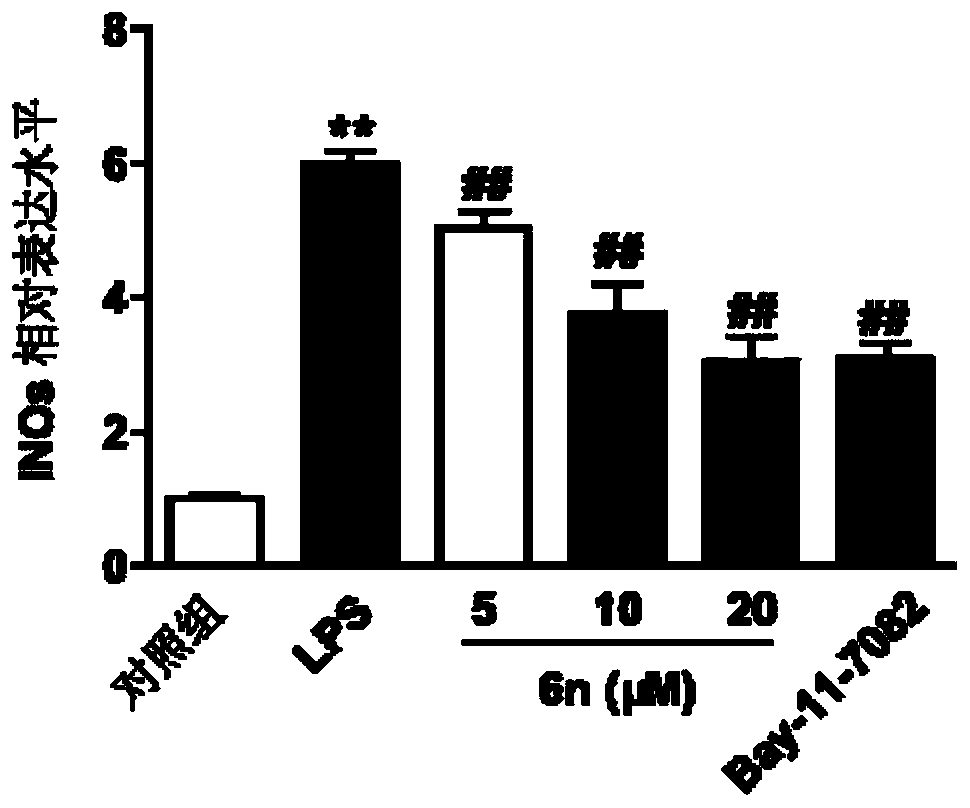
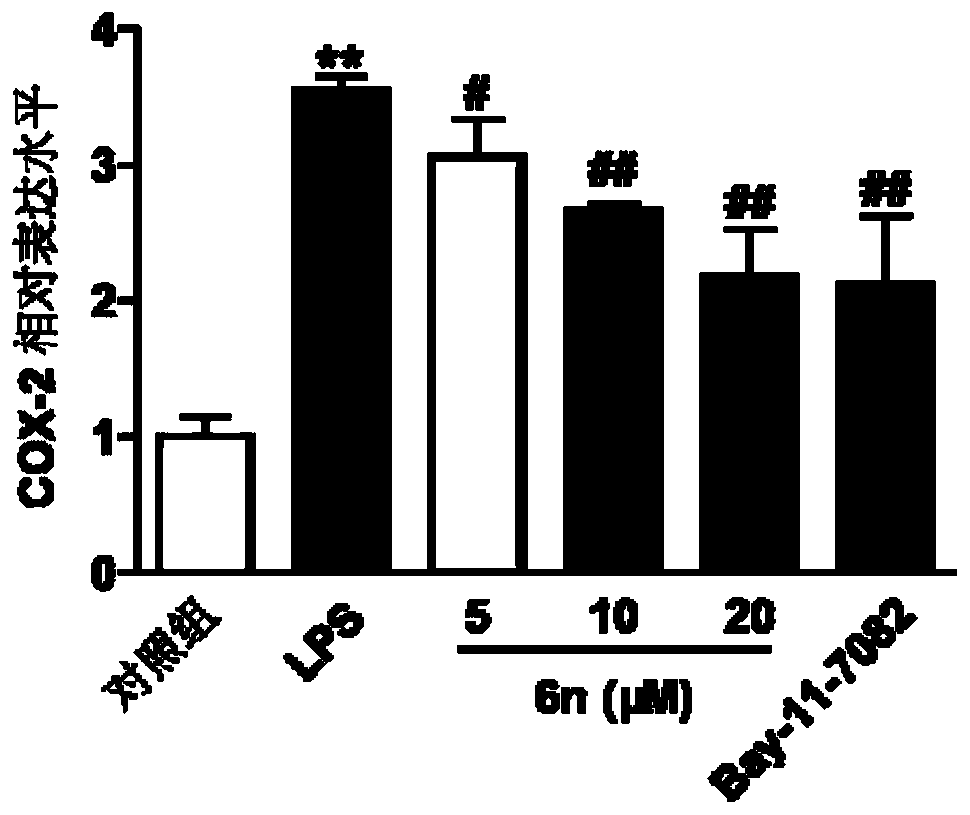
Benzyl piperazine compound with anti-inflammatory activity as well as preparation method and medical application thereof
A technology of benzylpiperazine and compounds, applied in the field of medicinal chemistry, can solve problems such as side effects and threats to the safety of patients' lives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Synthesis of 1-(2-(4-(4-chlorobenzyl)piperazine-1-carbonyl)phenyl)ethanone (4a)
[0078] Step 1: Synthesis of 2-vinylbenzoic acid (2)
[0079] In a dry 100mL round bottom flask, add methyltriphenylphosphonium ammonium bromide (7.15g, 20.0mmol) and potassium tert-butoxide (3.37g, 30.0mmol) successively, add anhydrous tetrahydrofuran (100.0mL) to dissolve, and react The solution was stirred at room temperature for 1 hour under nitrogen protection. Anhydrous THF solution of 2-formylbenzoic acid (1.5 g, 10.0 mmol) was slowly added dropwise to the reaction solution, and the reaction solution was refluxed and stirred for 12 hours. TLC [V (petroleum ether): V (ethyl acetate) = 2:1 as developing solvent] showed that the reaction was almost complete. 100 mL of saturated ammonium chloride solution was added to the reaction liquid. The reaction solution was transferred to a separatory funnel, the organic layer was separated, and the aqueous layer was extracted with dichlorometh...
Embodiment 2
[0085] Synthesis of 1-(2-(4-(4-methoxybenzyl)piperazine-1-carbonyl)phenyl)ethanone (4b)
[0086] Referring to the synthesis method of Example 1, using compound 3 (0.82g, 3mmol) and 1-(4-methoxybenzyl)piperazine (0.84g, 4 mmol) as raw materials, column chromatography purified to obtain 0.38g of a white solid , yield 35.9%. m.p.88.4~89.6℃. 1 H NMR (400 MHz, DMSO-d 6 )δ: 7.98 (dd, J=7.7, 1.2Hz, 1H, Ar-H), 7.63 (d, J=7.5, 1.3Hz, 1H, Ar-H), 7.55 (d, J=7.6, 1.4Hz, 1H,Ar-H),7.32-7.25(m,1H,Ar-H),7.25-7.18(m,2H,Ar-H),6.94–6.84(m,2H,Ar-H),3.73(s, 3H,CH 3 ), 3.57(d, J=5.1Hz, 2H, piperazine-H), 3.42(s, 2H, CH 2 ), 3.04(t, J = 5.0Hz, 2H, piperazine-H), 2.55(s, 3H, CH 3 ), 2.41(t, J=5.1Hz, 2H, piperazine-H), 2.26(t, J=5.0 Hz, 2H, piperazine-H). 13 C NMR (101MHz, DMSO-d 6 )δ: 199.22, 169.35, 158.78, 137.12, 135.80, 132.92, 130.54, 130.25, 130.12, 129.28, 127.41, 114.04, 61.78, 55.45, 52.47, 52.16, 45.75, 48-33.2 M+H] + .
Embodiment 3
[0088] Synthesis of 1-(2-(4-(4-cyanobenzyl)piperazine-1-carbonyl)phenyl)ethanone (4c)
[0089] Referring to the synthesis method of Example 1, using compound 3 (0.82 g, 3 mmol) and 1-(4-cyanobenzyl) piperazine (0.80 g, 4 mmol) as raw materials, column chromatography purified to obtain 0.48 g of a white solid, Yield 46.1%. m.p.143.6~145.6℃. 1 H NMR (400 MHz, DMSO-d 6 )δ: 7.98 (dd, J = 7.8, 1.2Hz, 1H, Ar-H), 7.80 (d, J = 8.2Hz, 2H, Ar-H), 7.64 (dd, J = 7.5, 1.3Hz, 1H, Ar-H), 7.59-7.49 (m, 3H, Ar-H), 7.29 (dd, J = 7.8, 1.2Hz, 1H, Ar-H), 3.60 (d, J = 4.4Hz, 4H, piperazine-H ,CH 2 ), 3.06(t, J=5.0Hz, 2H, piperazine-H), 2.56(s, 3H, CH 3 ), 2.46(t, J=5.1Hz, 2H, piperazine-H), 2.30(t, J=5.0Hz, 2H, piperazine-H); 13 C NMR (101MHz, DMSO-d 6 )δ: 199.22, 169.41, 144.61, 137.07, 135.70, 132.98, 132.65, 130.33, 130.01, 129.32, 127.40, 110.23, 61.60, 52.53, 52.29, 46.70, 41.58-0, 28.31.ESI H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com