Method for preparing blue-light fluorescent powder Sr2B5O9Cl:Eu<2+> by means of self-reduction
A fluorescent powder and blue light technology, applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of complex operation and high temperature of blue light fluorescent powder, and achieve the effect of fewer types, simple methods, and low preparation temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
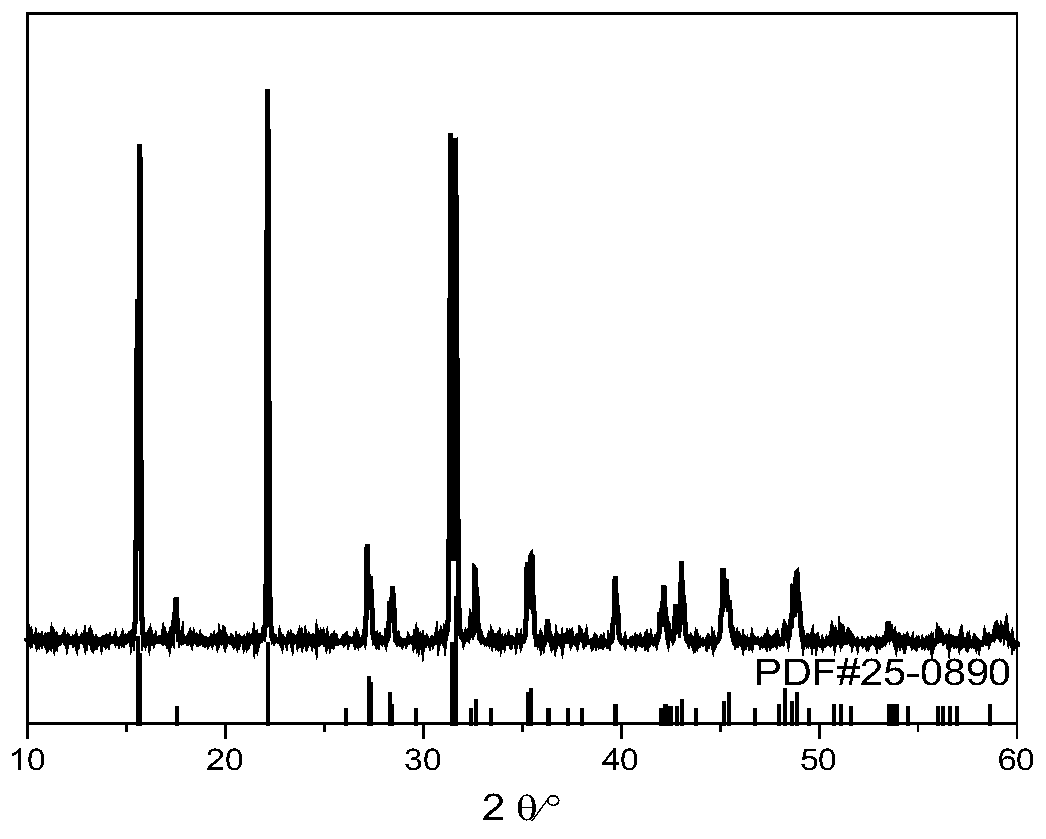
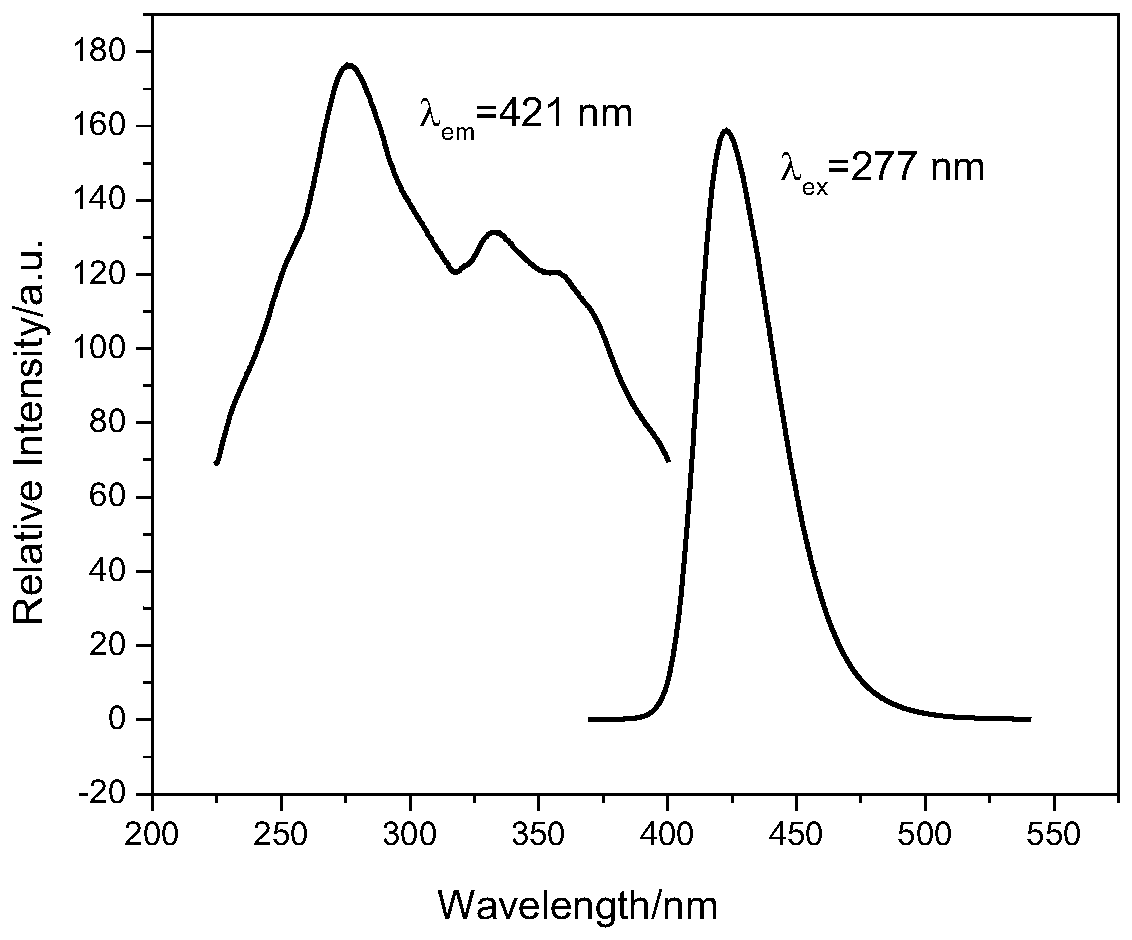
[0028] 2.66g (10mmol) SrCl 2 ·6H 2 O, 1.54g (25mmol) H 3 BO 3 and 0.108g (0.6mmol) Eu 2 o 3 Grind for 20 minutes to make the three fully mixed evenly, then place in a platinum crucible, seal the platinum crucible in a stainless steel flanged reactor (use temperature less than 500°C, use pressure less than 49bar), and place the reactor in a muffle furnace In the process, the heating rate was raised to 350°C at a rate of 5°C / min, the constant temperature was reacted for 12 hours, and naturally cooled to obtain the target product Sr 2 B 5 o 9 Cl:Eu 2+ (See figure 1 ). This phosphor emits blue light, and the maximum emission peak is at 421nm (see figure 2 ), the quantum yield is 77%.
Embodiment 2
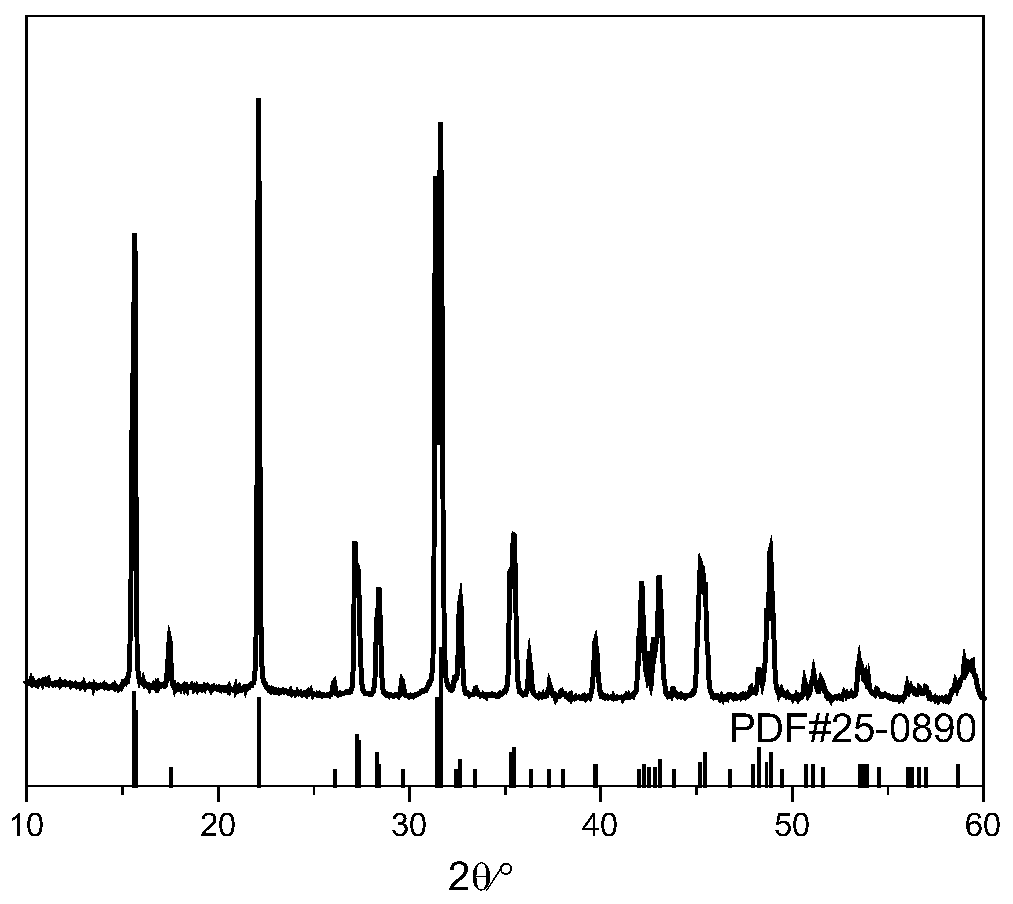
[0030] 2.66g (10mmol) SrCl 2 ·6H 2 O, 1.54g (25mmol) H 3 BO 3 and 0.144g (0.8mmol) Eu 2 o 3 Grind for 20 minutes to make the three fully mixed evenly, then place in a platinum crucible, seal the platinum crucible in a stainless steel flanged reactor (use temperature less than 500°C, use pressure less than 49bar), and place the reactor in a muffle furnace In the process, the heating rate was raised to 350°C at a rate of 5°C / min, the constant temperature was reacted for 12 hours, and naturally cooled to obtain the target product Sr 2 B 5 o 9 Cl:Eu 2+ (See image 3 ). This phosphor emits blue light, and the maximum emission peak is at 421nm (see Figure 4 ), the quantum yield is 84%.
Embodiment 3
[0032] 2.66g (10mmol) SrCl 2 ·6H 2 O, 1.54g (25mmol) H 3 BO 3 and 0.072g (0.4mmol) Eu 2 o 3 Grind for 20 minutes to make the three fully mixed evenly, then place in a platinum crucible, seal the platinum crucible in a stainless steel flanged reactor (use temperature less than 500°C, use pressure less than 49bar), and place the reactor in a muffle furnace In the process, the heating rate was raised to 350°C at a rate of 5°C / min, the constant temperature was reacted for 12 hours, and naturally cooled to obtain the target product Sr 2 B 5 o 9 Cl:Eu 2+ (See Figure 5 ). This phosphor emits blue light, and the maximum emission peak is at 421nm (see Figure 6 ), the quantum yield is 72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com