Synthesis and photoelectric performance research of carbazole room-temperature phosphorescent material containing S/Se/Te heavy atoms
A carbazole-based and compound technology, applied in the field of synthesis of carbazole-based room temperature phosphorescent materials, can solve problems such as self-quenching and oxygen quenching, and achieve the effects of mild synthesis conditions, narrow optical band gap, and high electron mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
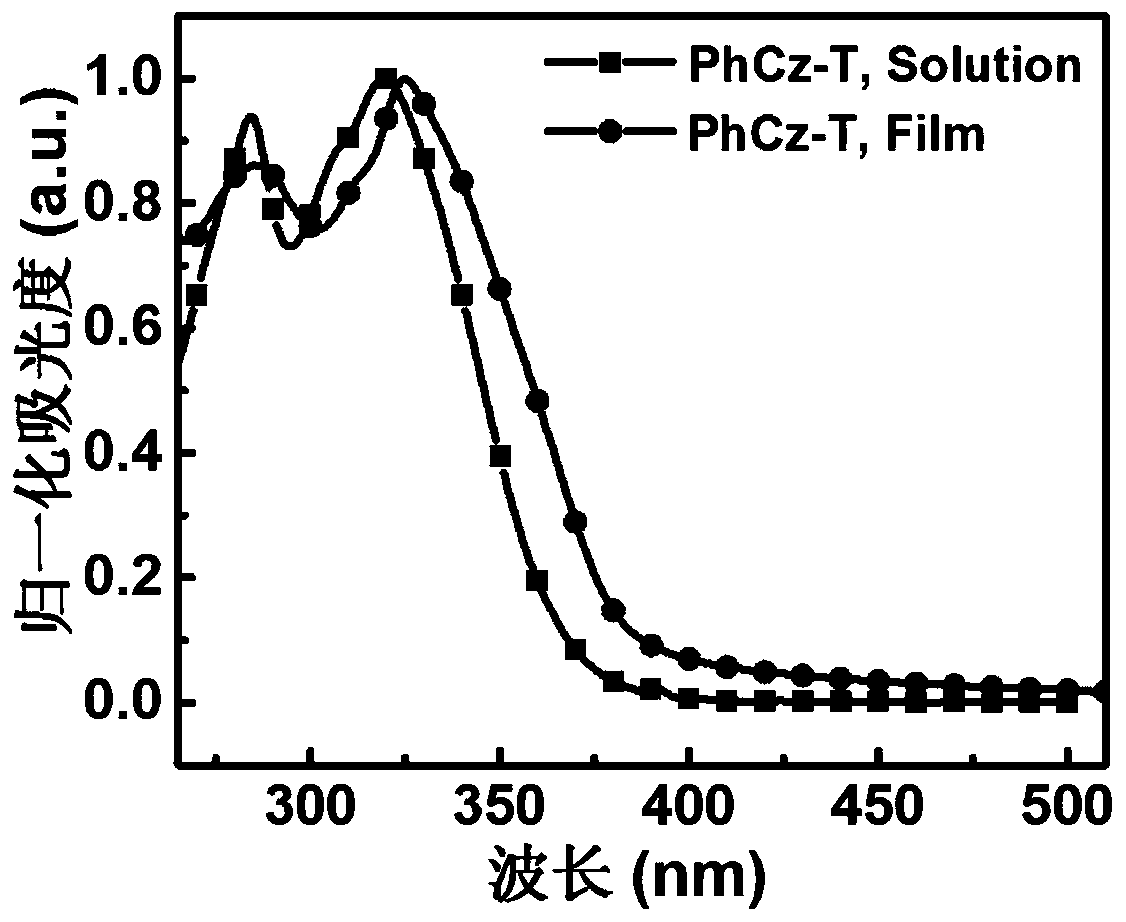
[0054] A D-A type carbazolyl compound PhCz-T of the present invention has a general structural formula of formula I:
[0055]
[0056] The synthetic route of PhCz-T is:
[0057]
[0058] Specifically include the following steps:
[0059] (1) Synthesis of intermediate a: Add carbazole (1.0 g, 6.0 mmol) and anhydrous tetrahydrofuran (30 mL) to 100 mL of Schlenk under nitrogen protection, and then place the reaction bottle in a cryogenic apparatus at -78 °C. At -78°C, lithium diisopropylamide (2.0M, 3.3mL) was slowly added dropwise into the reaction flask, reacted at -78°C for 1h, and then slowly returned to -40°C and stirred for 1h. Then, a tetrahydrofuran solution (2.4 g, 9 mmol) of 4-iodobenzoyl chloride was injected once at −78° C., and finally stirred overnight at room temperature. The mixture was extracted with ethyl acetate and water, dried, filtered and spin-dried. The crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate ...
Embodiment 2
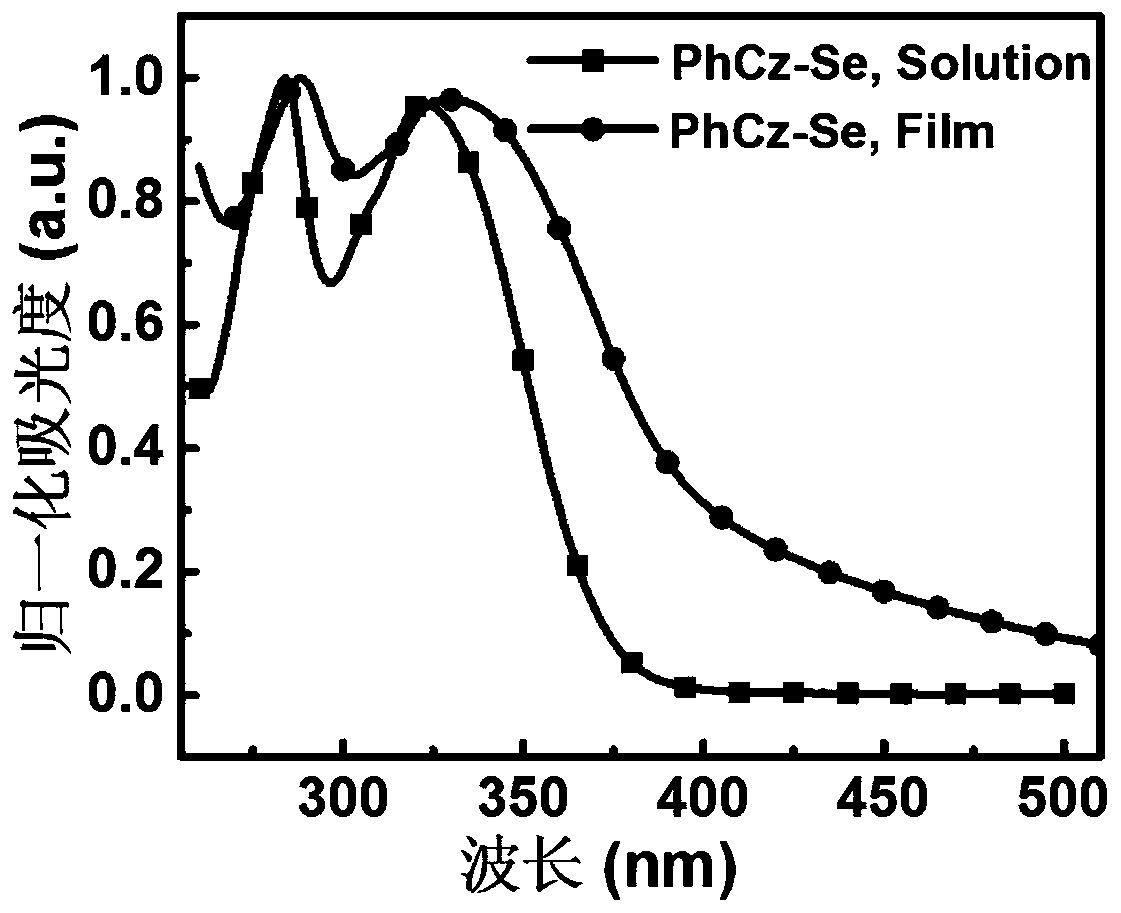
[0076] A D-A type carbazolyl compound PhCz-Se of the present invention has a general structural formula of formula II:
[0077]
[0078] The synthetic route of PhCz-Se is:
[0079]
[0080] Concrete synthetic steps are:
[0081] (1) Synthesis of intermediate a: synthesized with reference to the synthetic method of the above-mentioned Example 1.
[0082] (2) Synthesis of compound PhCz-Se: In a 10 mL microwave bottle, add iodocarbazolyl intermediate (0.85 g, 2.1 mmol), selenophene unilateral tin compound (1.0 g, 3.4 mmol) and 6 mL of toluene. Oxygen was replaced under nitrogen for 20 minutes, then the catalyst tetrakistriphenylphosphine palladium (8 mg) was added, nitrogen was blown for 10 minutes, a microwave cover was sealed, and the reaction was carried out at 120° C. for 4 hours. After cooling to room temperature, extract with dichloromethane, dry over anhydrous magnesium sulfate, filter with suction, and spin dry. The mixture was purified by silica gel column chrom...
Embodiment 3
[0092] A D-A type carbazolyl compound PhCz-Te of the present invention has a general structural formula of formula III:
[0093]
[0094] The synthetic route of PhCz-Te is:
[0095]
[0096] Concrete synthetic steps are:
[0097] (1) Synthesis of intermediate a: synthesized with reference to the synthetic method of the above-mentioned Example 1.
[0098] (2) Synthesis of compound PhCz-Te: In a 10mL microwave bottle, put iodocarbazolyl intermediate (0.8g, 2.0mmol), tellurphene unilateral tin compound (1.0g, 3.0mmol) and 6mL chlorobenzene . Oxygen was replaced under nitrogen for 20 minutes, then the catalyst tetrakistriphenylphosphine palladium (8 mg) was added, nitrogen was blown for 10 minutes, a microwave cover was sealed, and the reaction was carried out at 140° C. for 4 hours. After cooling to room temperature, extract with dichloromethane, dry over anhydrous magnesium sulfate, filter with suction, and spin dry. The mixture was purified by silica gel column chroma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com