Recombinant L-asparaginase and preparation method and use thereof
A technology of asparaginase and DNA sequence, applied in biochemical equipment and methods, recombinant DNA technology, enzymes, etc., can solve the problem of side effects and short half-life of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
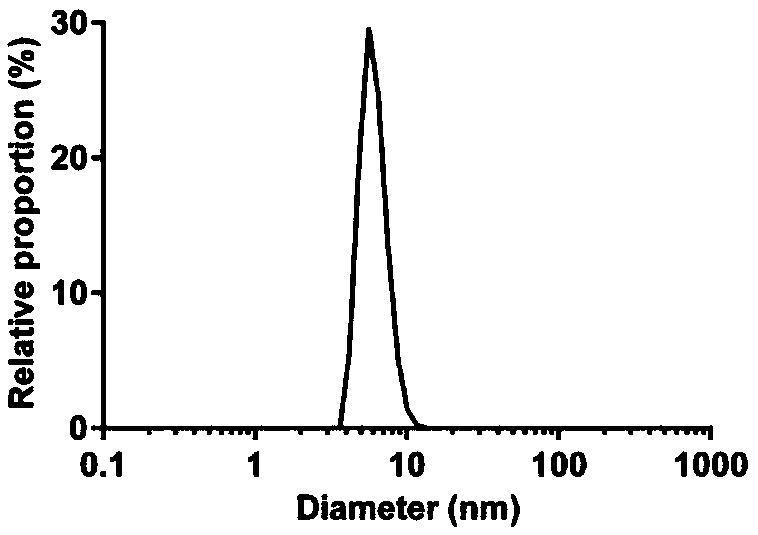
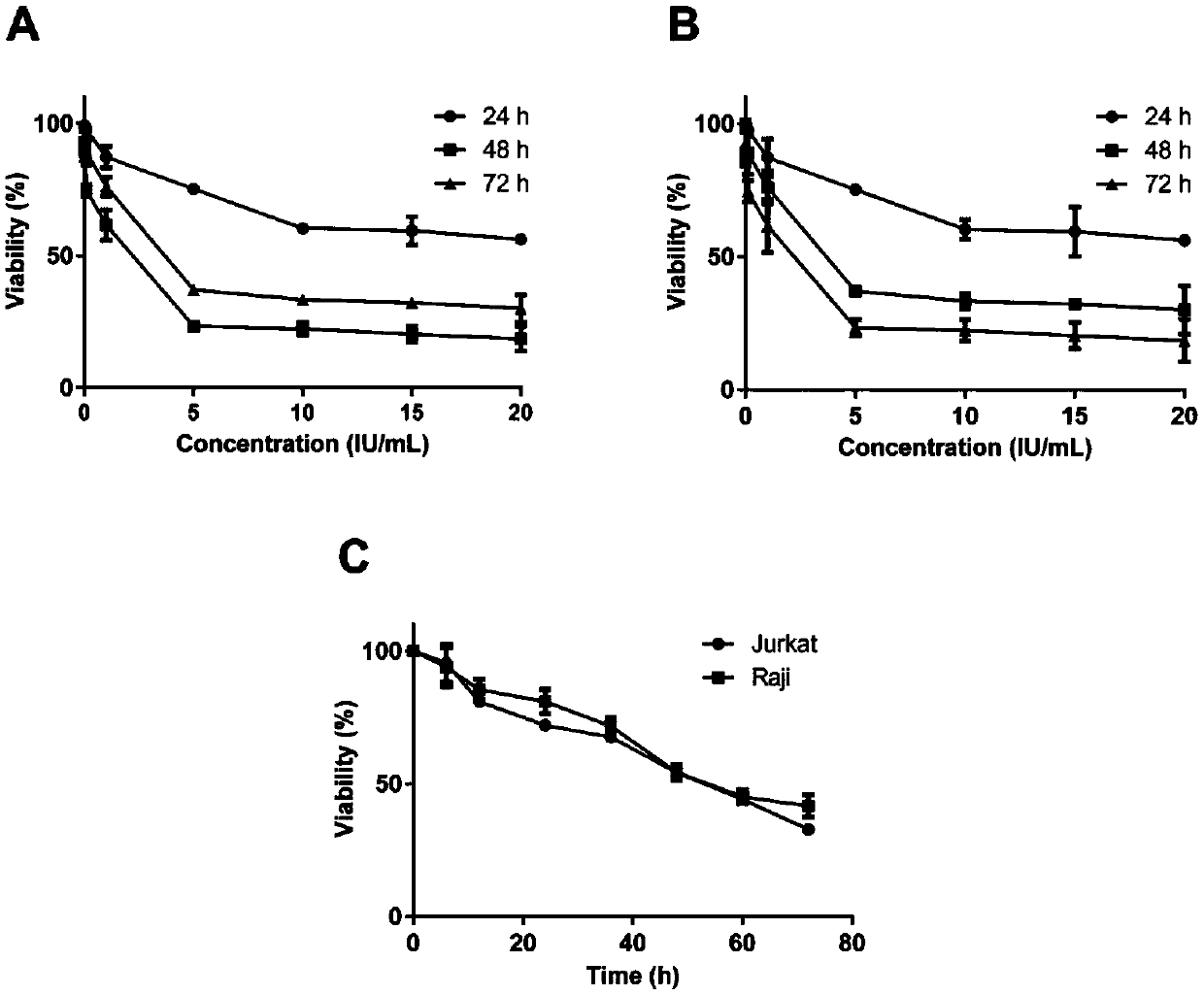
[0014] Example Expression and purification, property determination, activity research and in vitro safety research of recombinant L-asp
[0015] 1 Materials and methods
[0016] 1.1.1 Cells, plasmids and strains
[0017] The prokaryotic expression vector pET-22b, Escherichia coli clone strain Top10, and Escherichia coli expression strain BL21(DE3) are all preserved by our laboratory. Jurkat and HBE cells were purchased from the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, and Raji cells were provided by the Blood Center of Soochow University.
[0018] 1.1.2 Reagents
[0019] Tsi High-Fidelity DNA polymerase was purchased from Tianjin Jinbeisi Biotechnology Co., Ltd.; Tsi 4 Ligase was purchased from American Thermo Company; restriction endonucleases NdeI and XhoI were purchased from American Thermo Company; plasmid mini-extraction kit was purchased from Shanghai Jierui Bioengineering Co., Ltd.; DNA gel ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com