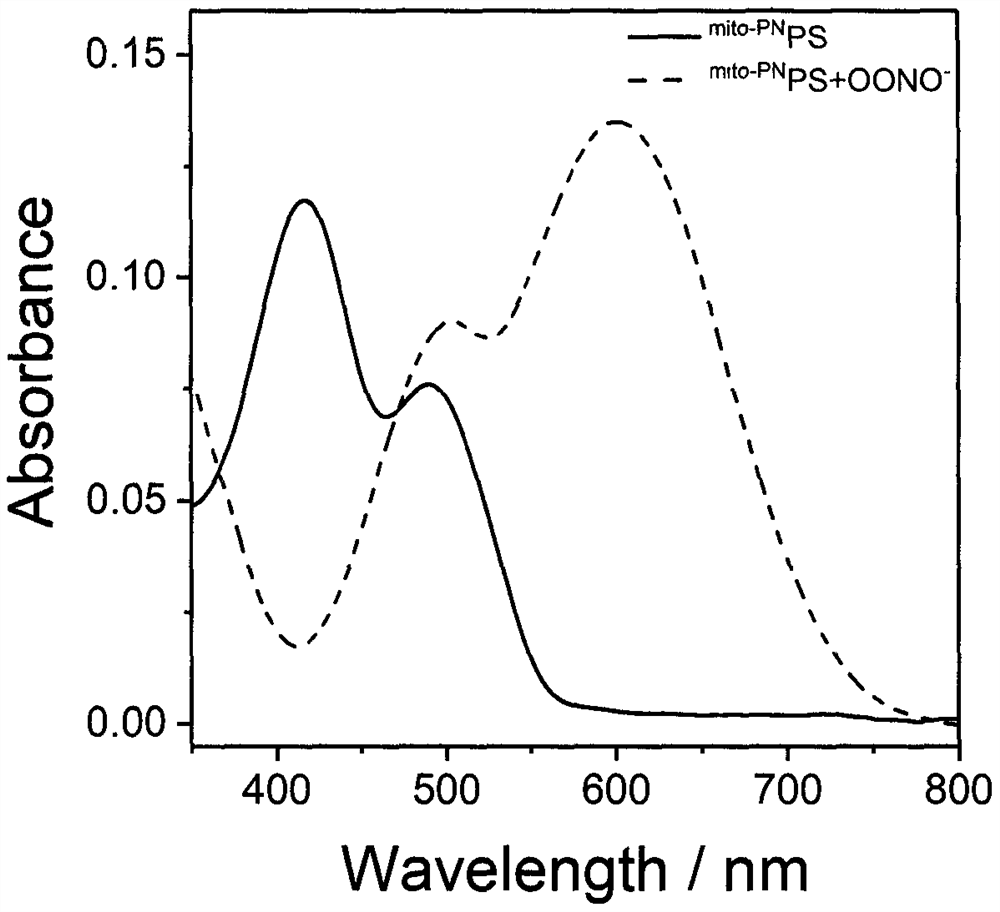
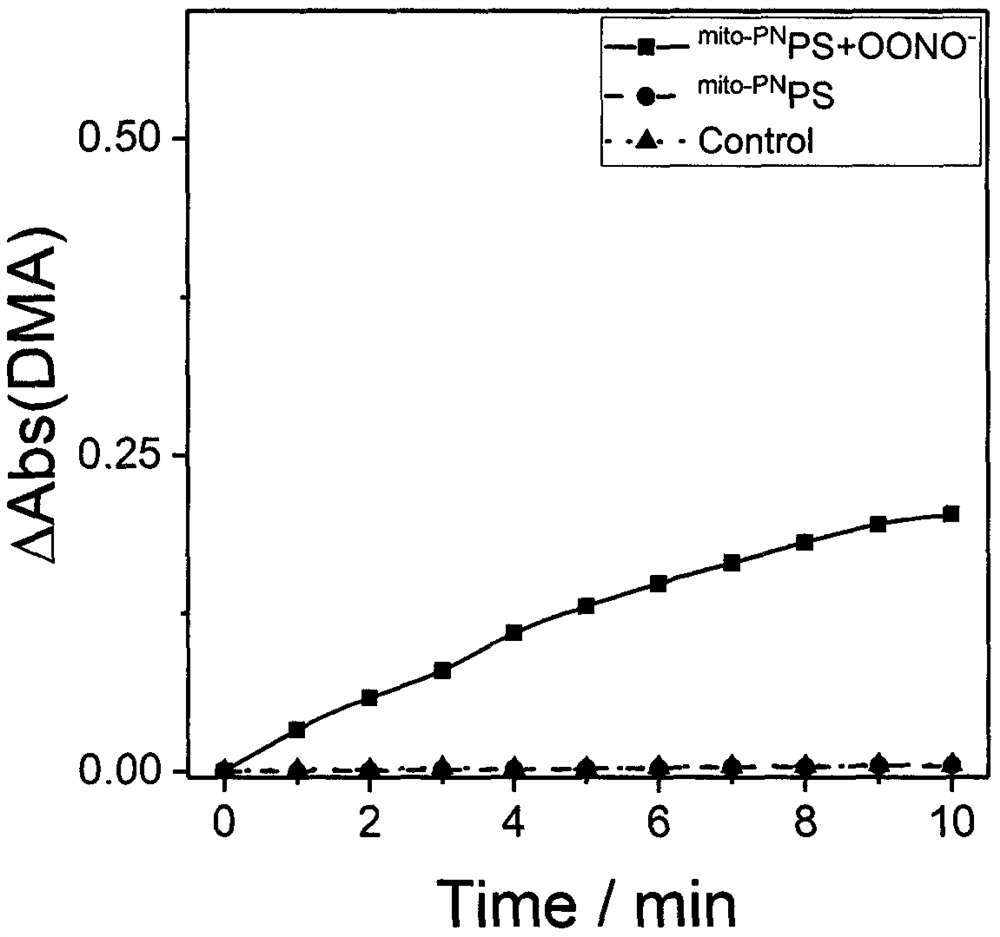
Preparation and application of a mitochondria-targeted stimulus-responsive photosensitizer
A stimuli-responsive, mitochondrial technology, applied in medical preparations containing active ingredients, photodynamic therapy, wave energy or particle radiation treatment materials, etc., can solve the problem of not having switch controllability and reducing the quantum yield of singlet oxygen and other issues to achieve the effect of improving the photodynamic ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
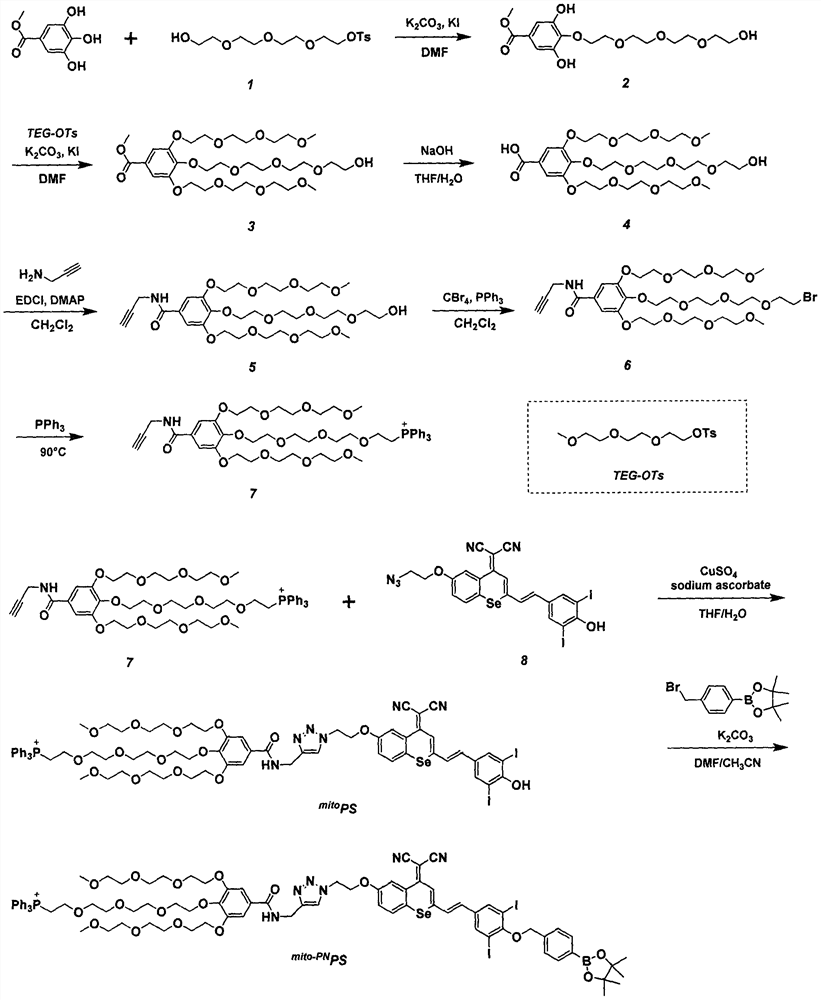
[0024] Example 1: Synthesis of Compound 7, the synthetic route is attached figure 1
[0025] Methyl gallate (3.0g, 16.3mmol) was dissolved in dry DMF (15mL), potassium carbonate (4.5g, 32.6mmol) and potassium iodide (0.27g, 1.63mmol) were added, stirred at 80°C for 10min, dropwise A dry DMF (5 mL) solution containing compound 1 (5.68 g, 16.3 mmol) was added dropwise and stirred overnight at this temperature. After the reaction, the solvent was removed by rotary evaporation, the mixture was dissolved in ethyl acetate, washed 3 times with saturated brine, dried over anhydrous sodium sulfate, the solvent was removed by rotary evaporation, and column chromatography was performed with petroleum ether and ethyl acetate as eluents. Compound 2 (1.82 g, 31.0%) was obtained as a colorless oily substance. 1 H NMR (400MHz, CDCl 3 )δ (ppm) 7.63 (s, 2H), 7.15 (s, 2H), 4.20-4.14 (t, J=3.2Hz, 2H), 3.85 (s, 3H), 3.74 (m, 8H), 3.69 (s , 4H), 3.62(t, J=4.4Hz, 2H), 3.48-3.38(s, 1H). 13 C NMR...
Embodiment 2
[0031] Embodiment two: synthesis mito-PN PS, the synthetic route is attached figure 1
[0032] Compound 7 (129mg, 0.14mmol), azide P.S. Se-I (100mg, 0.14mmol), sodium ascorbate (2.8mg, 0.014mmol) and anhydrous copper sulfate (2.2mg, 0.0014mmol) were dissolved in a mixed solution of tetrahydrofuran / water (4mL / 1mL), and reacted at room temperature for 24h. After the reaction, extract with dichloromethane, dry over anhydrous sodium sulfate, remove the solvent by rotary evaporation, and use dichloromethane and methanol as eluents for column chromatography to obtain a black-green solid compound mito PS (0.15 g, 65.6%). 1 H NMR (400MHz, CDCl 3 )δ (ppm) 8.99 (s, 1H), 8.17 (s, 1H), 8.08 (s, 1H), 7.81-7.56 (m, 18H), 7.53-7.41 (m, 2H), 7.34 (s, 2H) , 7.13(d, J=8.8Hz, 1H), 6.75(d, J=15.6Hz, 1H), 6.64(d, J=16.0Hz, 1H), 4.86-4.66(m, 4H), 4.45(s, 2H), 4.15(m, 6H), 3.92-3.71(m, 10H), 3.66(m, 4H), 3.64-3.53(m, 10H), 3.50(m, 4H), 3.38-3.30(m, 8H) , 3.26(m, 2H), 3.21(m, 2H). 13 C NMR (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com