Medicine compound preparation for treating osteoarthritis and preparation method of medicine compound preparation
A compound preparation and osteoarthritis technology, applied in the field of biomedicine, can solve the problems of discomfort, joint swelling, large injection dose, etc., and achieve the effect of avoiding joint swelling, slowing down the progression of osteoarthritis, and preventing and developing osteoarthritis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
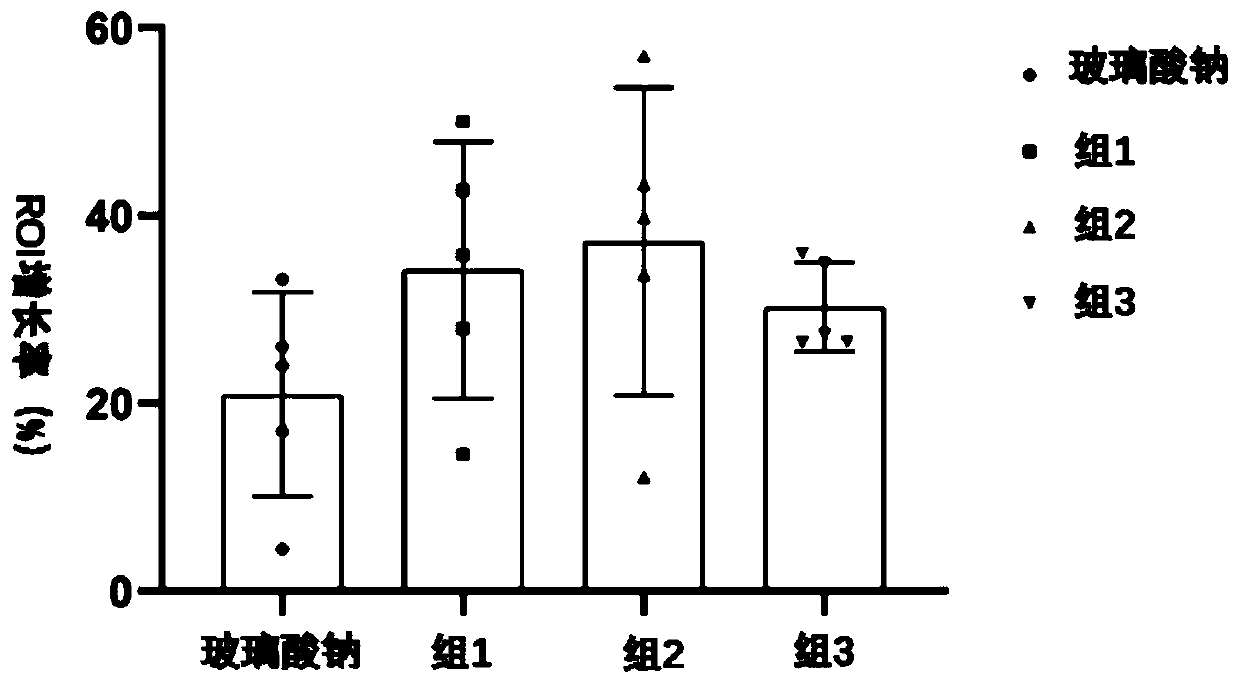
[0068] The preparation of embodiment 1 compound injection
[0069] S1: To make solution, take 40 μg of Sema 3a human recombinant protein and dissolve it in 10 mL of sodium hyaluronate injection, vortex fully to dissolve, and make Sema 3a-sodium hyaluronate compound injection with a Sema 3a protein concentration of 4 μg / mL;
[0070] S2: Activated carbon treatment, add 0.2% of the total amount of the solution to the medicinal liquid and use activated carbon adsorption treatment;
[0071] S: 3: primary filtration, filter the liquid medicine with filter paper;
[0072] S4: Sterilize by filtration, use a microporous filter membrane with a pore size of 0.22 μm to sterilize the medicinal solution and remove the heat source;
[0073] S5: Subpackaging the medicinal solution in units of 2 mL, filling and sealing, to obtain an injection solution.
Embodiment 2
[0074] The preparation of embodiment 2 compound injection
[0075] The preparation method was the same as that in Example 1, except that in step S1, 60 μg of Sema 3a human recombinant protein was dissolved in 10 mL of sodium hyaluronate injection, and fully shaken to dissolve, so that the Sema 3a protein concentration was 6 μg / mL.
Embodiment 3
[0076] The preparation of embodiment 3 compound injections
[0077] The preparation method was the same as that in Example 1, except that in step S1, 90 μg of Sema 3a human recombinant protein was dissolved in 10 mL of sodium hyaluronate injection, and fully shaken to dissolve, so that the Sema 3a protein concentration was 9 μg / mL.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com