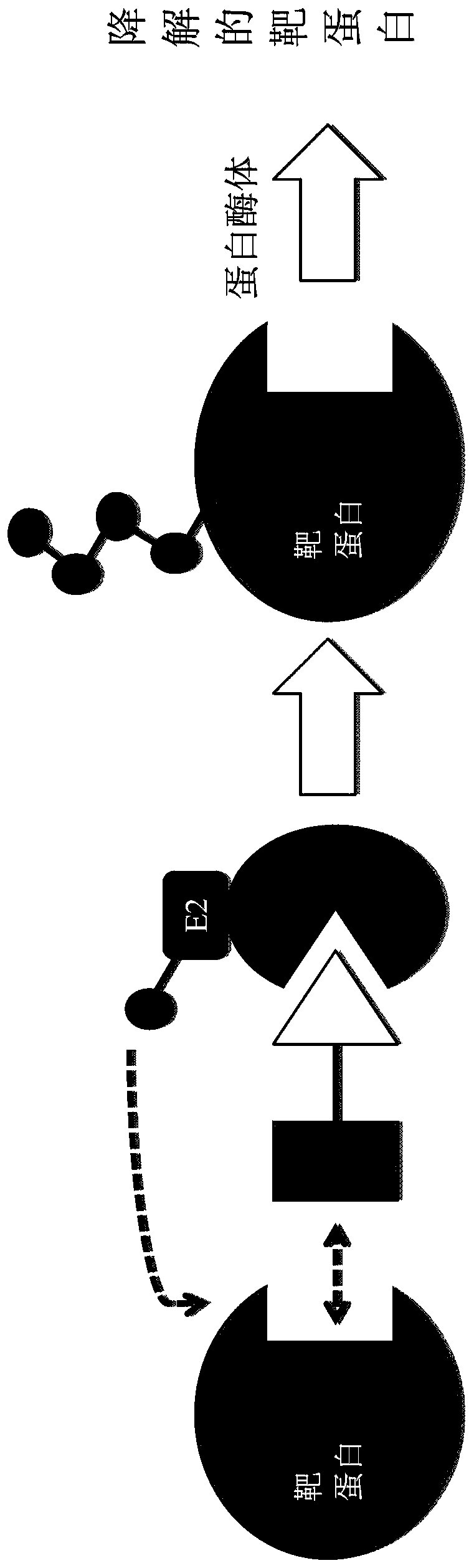
EGFR proteolysis targeting chimeric molecules and associated methods of use
A technology for linking moieties and bifunctional compounds, applied in EGFR proteolysis targeting chimeric molecules and related fields of use, can solve problems such as uncertain accessibility, and achieve the effect of broad-spectrum pharmacological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
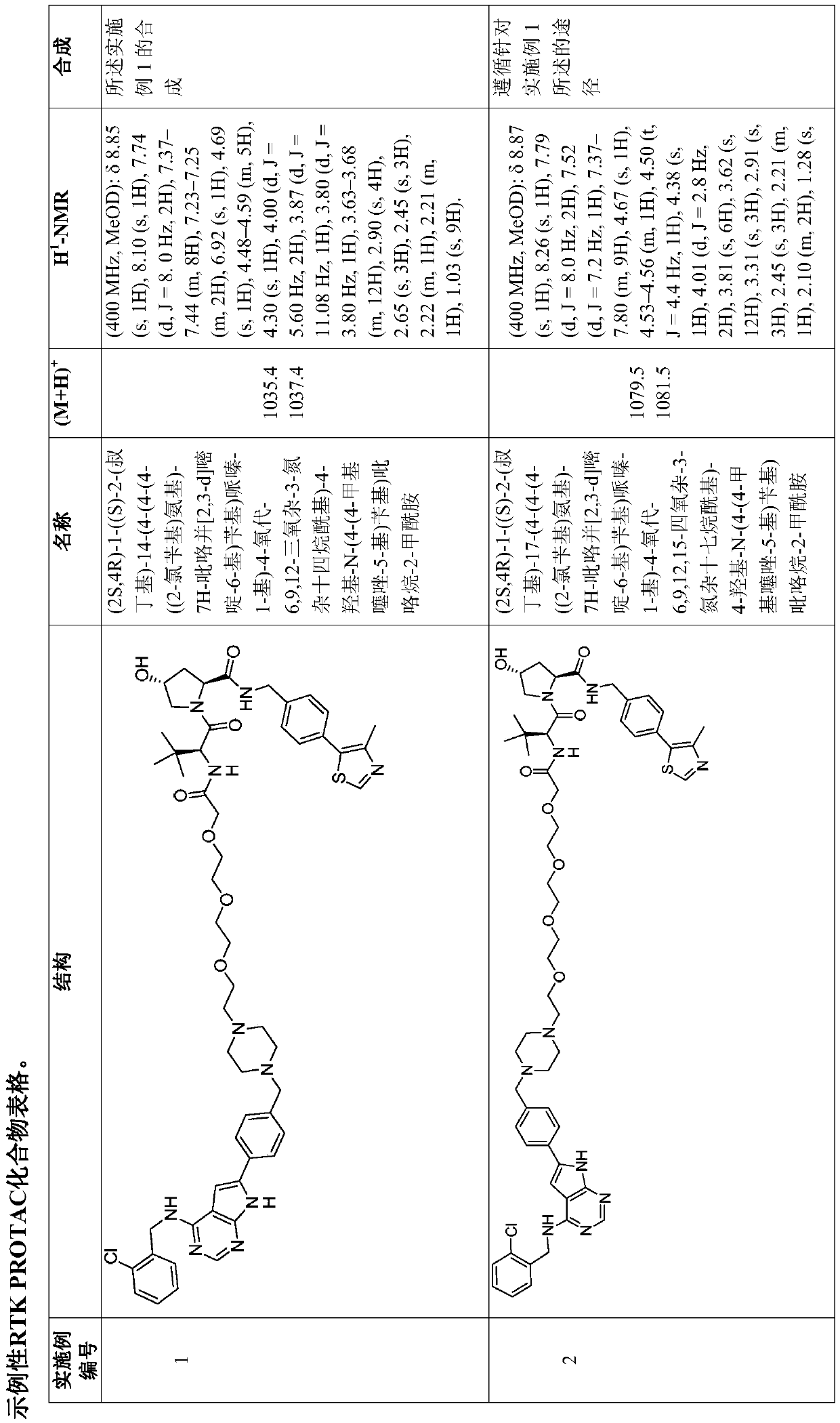
Embodiment 1
[1894] Synthesis of Example 1
[1895] (2S,4R)-1-((S)-2-(tert-butyl)-14-(4-(4-(4-((2-chlorobenzyl)amino)-7H-pyrrolo[2, 3-d]pyrimidin-6-yl)benzyl)piperazin-1-yl)-4-oxo-6,9,12-trioxa-3-azatetradecanoyl)-4-hydroxyl- N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide
[1896]
[1897] Synthesis scheme:
[1898]
[1899] 1. Step – Synthesis of tert-butyl 2-(2-(2-(2-((methylsulfonyl)oxy)ethoxy)ethoxy)ethoxy)acetate
[1900]
[1901] To tert-butyl 2-(2-(2-(2-hydroxyethoxy)ethoxy)ethoxy)acetate (300.0mg, 1.13mmol) and TEA (344.5mg, 3.40mmol) at 0°C To a solution of DCM (5 mL) was added MsCl (195.8 mg, 1.70 mmol). The solution was stirred at room temperature for 1 hour. The mixture was quenched with water, then extracted with DCM (10 mL x 3). The combined organic layers were washed with brine (15 mL x 3). The organic phase was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give the crude title compound 2-(2-(2-(2-((meth...
Embodiment 8
[1917] Synthesis of Example 8
[1918] 4-((17-(4-(4-(4-((2-chlorobenzyl)amino)-7H-pyrrolo[2,3-d]pyrimidin-6-yl)benzyl)piperazine-1 -yl)-3,6,9,12,15-pentaoxahetadecyl)amino)-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3 - dione
[1919]
[1920] Synthesis scheme:
[1921]
[1922] 1. Step – 2-(2,6-Dioxopiperidin-3-yl)-4-((17-Hydroxy-3,6,9,12,15-pentaoxahetadecyl)amino) Synthesis of Isoindoline-1,3-dione
[1923]
[1924] To 17-amino-3,6,9,12,15-pentoxahetadecan-1-ol hydrochloride (2.00g, 7.1mmol) and 2-(2,6-dioxopiperidine-3 To a solution of -yl)-4-fluoroisoindoline-1,3-dione (2.94 g, 10.65 mmol) in NMP (10 mL) was added DIEA (3.67 g, 28.4 mmol). The solution was stirred at 90°C for 2.5 hours. It was then cooled to room temperature and quenched with water (20 mL). The mixture was extracted with DCM (50 mL x 3). The combined organic layers were dried over anhydrous sodium sulfate, filtered and concentrated in vacuo. The residue was purified by silica gel column ch...
Embodiment 10
[1932] Synthesis of Example 10
[1933]
[1934]4-((2-(2-(2-(2-(4-(4-((2-chlorobenzyl)amino)-7H-pyrrolo[2,3-d]pyrimidin-6-yl)benzene Oxy)ethoxy)ethoxy)ethoxy)ethyl)amino)-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione
[1935] Synthesis scheme:
[1936]
[1937] 1. Step – 6-(4-(2-(2-(2-(2-(N,N-di-tert-butoxycarbonyl-amino)ethoxy)ethoxy)ethoxy)ethoxy )Phenyl)-N-(2-chlorobenzyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine synthesis
[1938]
[1939] At 25°C, to 2-(2-(2-(2-(N,N-di-tert-butoxycarbonyl-amino)aminoethoxy)ethoxy)ethoxy)ethyl methanesulfonate (490mg, 1.04mmol) in DMF (10mL) and 4-(4-((2-chlorobenzyl)amino)-7H-pyrrolo[2,3-d]pyrimidin-6-yl)phenol (364mg, 1.04mmol) added K 2 CO 3 (430 mg, 3.12 mmol). The resulting solution was stirred at 70 °C for 16 hours. The resulting solution was cooled to 20 °C. with H 2 O (40 mL) diluted the mixture. The mixture was extracted with EtOAc (40 mL x 2). The combined organic layers were dried over anhydrous sodium sulfate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com