Antitumor nanometer medicine and preparation method thereof
A nano-drug, anti-tumor technology, applied in the field of medicine, to achieve good tumor suppression effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Such as figure 1 As shown, the first aspect of the present invention is a preparation method of anti-tumor nano-medicine, which specifically includes the following steps: using Fmoc-solid-phase synthesis method to introduce CRB into the capping of short peptide X-YpYY, and synthesize short peptide CRB-YpYY;
[0033] Using the method of polypeptide self-assembly, add PBS solution to the short peptide CRB-YpYY, and use carbonate solution to adjust the pH to 7.0~8.0; after that, add 1U / mL~3U / mL alkaline phosphatase to catalyze it Store overnight under the condition of ℃~6℃ to obtain the anti-tumor nano-medicine.
[0034] It should be noted that the Fmoc-solid-phase synthesis method is used to introduce CRB at the end of the short peptide X-YpYY, and the synthesis of the short peptide CRB-YpYY includes: introducing the short peptide X-YpYY at the second phosphorylation site into the CRB, to synthesize the short peptide CRB-YpYY
[0035] Specifically, the first step: using...
Embodiment 2
[0079] The reference compound CRB-pYYY(pY1) was synthesized according to the preparation method in Example 1, and the specific steps will not be repeated, and reference is made to the above description.
[0080] The first step, synthetic reference compound CRB-pYYY (pY1) with Fmoc-solid-phase synthesis method, structural formula is as follows:
[0081]
[0082] The characterization data for this structure are as follows:
[0083]1H NMR (300MHz, DMSO) δ7.16 (d, J = 8.4Hz, 1H), 7.09–6.83 (m, 4H), 6.58 (dd, J = 12.2, 5.8Hz, 3H), 4.40 (s, 2H) ,2.98(s,1H),2.82(d,J=27.4Hz,2H),2.66(s,1H),2.26(s,1H),1.97(s,1H),1.56(s,1H).
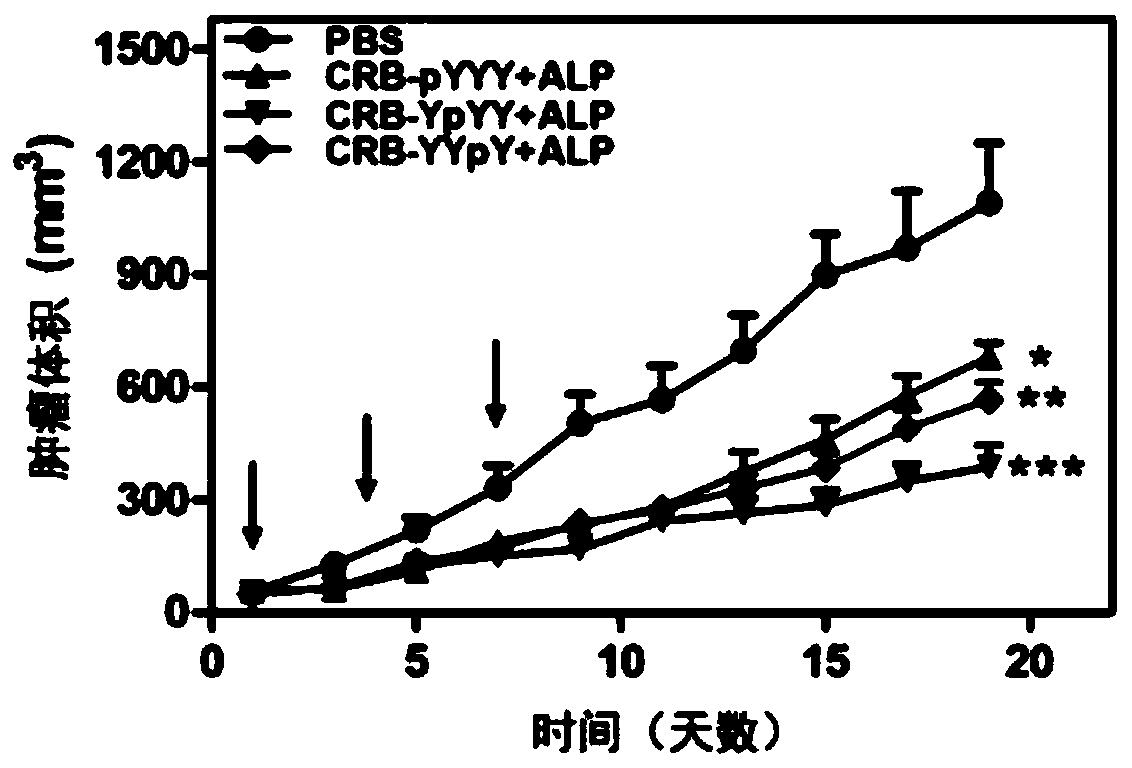
[0084] In the second step, take 5mg of the short peptide CRB-pYYY and place it in a 4mL glass bottle, then add PBS solution (pH=7.4) to make up to a total volume of 1mL, and adjust the pH value to 7.4 with 1M sodium carbonate solution. Mix well and sonicate at room temperature to dissolve completely, add 1U / mL alkaline phosphatase and store at 4°C overnight to...
Embodiment 3
[0088] The reference compound CRB-YYpY(pY3) was synthesized according to the preparation method of Example 1, the specific steps will not be described in detail, refer to the above description.
[0089] The specific structural formula is as follows:
[0090]
[0091] The characterization data for this structure are as follows:
[0092] 1 H NMR (300MHz, DMSO) δ7.16 (d, J = 8.4Hz, 1H), 7.09–6.83 (m, 4H), 6.58 (dd, J = 12.2, 5.8Hz, 3H), 4.40 (s, 2H) ,2.98(s,1H),2.82(d,J=27.4Hz,2H),2.66(s,1H),2.26(s,1H),1.97(s,1H),1.56(s,1H).
[0093] In the second step, take 5mg of the short peptide CRB-YYpY and place it in a 4mL glass bottle, then add PBS solution (pH=7.4) to make up to a total volume of 1mL, and adjust the pH value to 7.4 with 1M sodium carbonate solution. Mix well and sonicate at room temperature to dissolve completely, add 1U / mL alkaline phosphatase and store at 4°C overnight to obtain a nanostructure drug self-assembled by the polypeptide at the third phosphorylation s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com