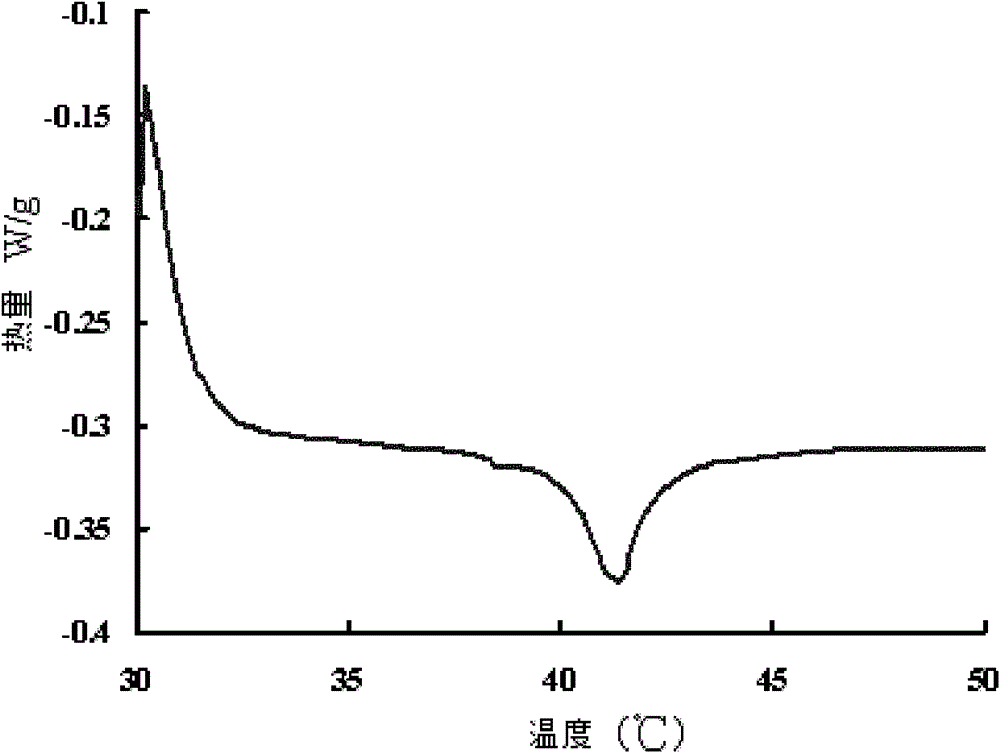
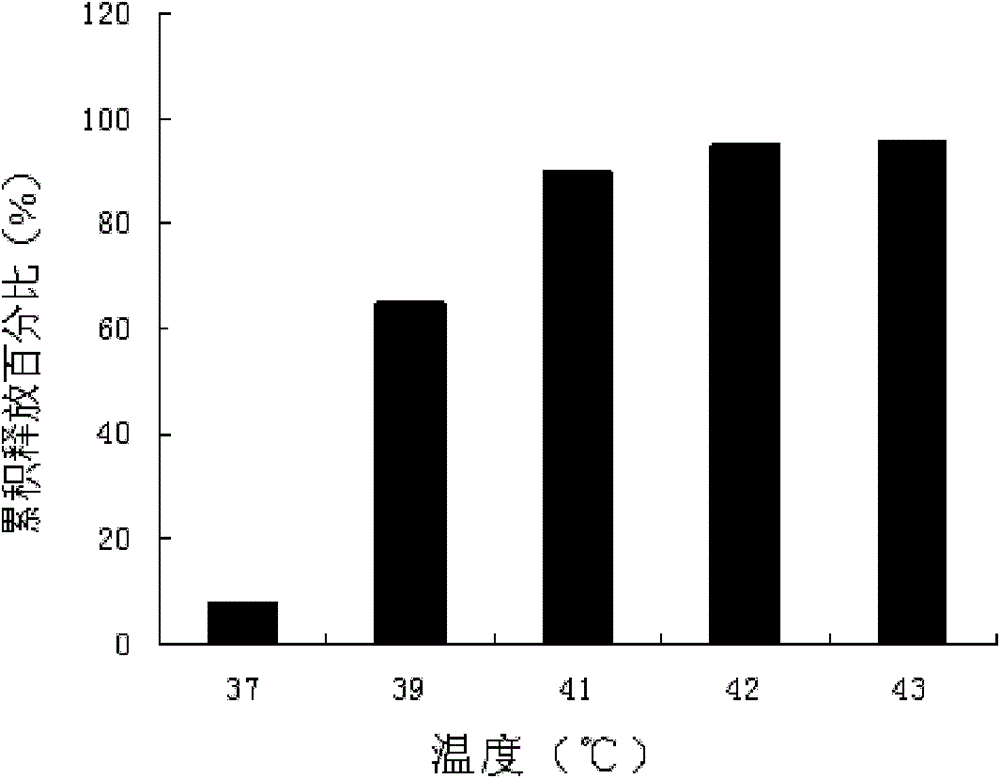
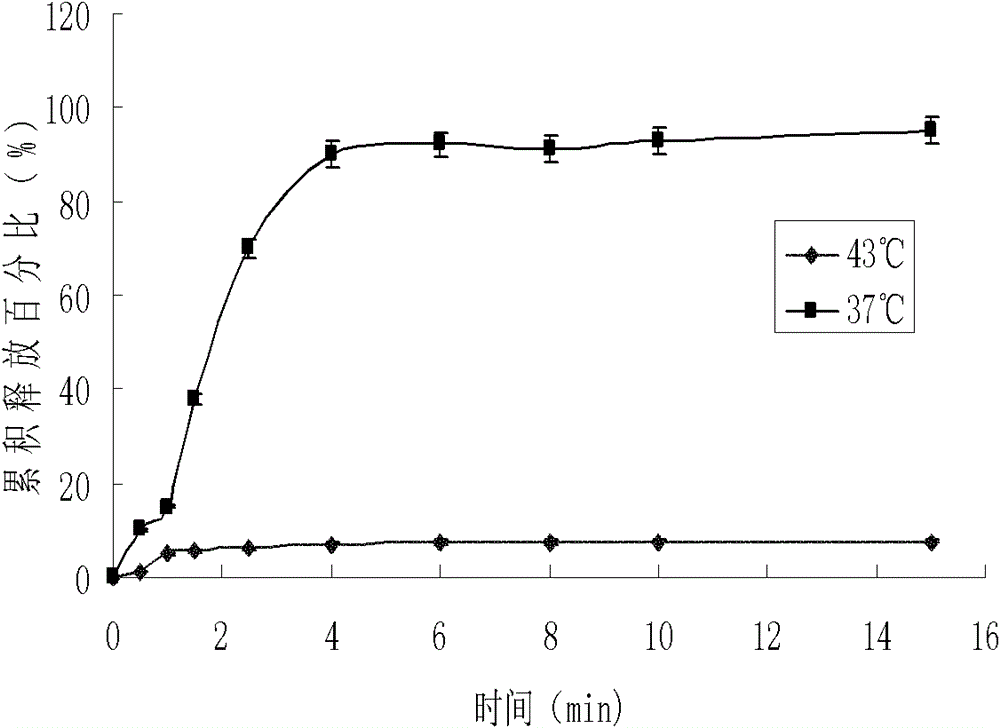
Thermosensitive liposome preparation of vinblastine drugs and preparation method thereof
The technology of liposome preparation and vinblastine, which is applied in the directions of liposome delivery, pharmaceutical formulation, and drug combination, can solve the problems of not significantly improving the curative effect, and achieves good targeting, high encapsulation rate, and not easy to leak. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of vinorelbine long-circulation thermosensitive liposome formulation
[0043] (1) Preparation of blank liposomes
[0044] Medicines and reagents needed: dipalmitoylphosphatidylcholine (DPPC), DSPE-mPEG2000, monostearylphosphorylcholine (MSPC), citric acid, trisodium citrate, sodium carbonate, double distilled water.
[0045] Table 1
[0046] Phosphate ester
DPPC
DSPE-mPEG2000
MSPC
Mass (g)
8.9
0.8
0.3
[0047] Weigh various phospholipids (the amount used to prepare 100ml of blank liposomes) according to Table 1, dissolve them in 80ml of chloroform, and remove organic solvents by rotary evaporation at 50-60°C to obtain a dry and uniform film attached to the bottom of the glass bottle. It was hydrated with 100ml, 0.2M, pH4 citrate buffer to obtain a multilamellar liposome solution. The polycarbonate membrane was extruded, first through the polycarbonate membrane with a pore size of 200 nm, and then through the polycarbonate membrane with a pore si...
Embodiment 2
[0049] Example 2 Preparation of Vinorelbine Long-Circulation Thermosensitive Liposome
[0050] (1) Preparation of blank liposomes
[0051] Same as Example 1.
[0052] (2) Preparation of drug-loaded liposomes by pH gradient method: Weigh 0.3g of vinorelbine and dissolve it in 100ml, 0.2M, pH4 citrate buffer solution to prepare a 3mg / ml drug solution. Take 20ml of the drug solution and add it to 20ml of blank liposome solution (it can be considered that the dosage of the drug is about 0.06g), add sodium carbonate solution at 25°C to adjust the pH of the external phase to 7.45, and load the drug for 60 minutes. Add sucrose (10%), a cryoprotectant, and place in a refrigerator at -20°C for cryopreservation.
Embodiment 3
[0053] Example 3 Preparation of Vinorelbine Long-Circulation Thermosensitive Liposome
[0054] (1) Preparation of blank liposomes
[0055] Medicines and reagents needed: dipalmitoylphosphatidylcholine (DPPC), DSPE-mPEG2000, distearoylphosphatidylglycerol (DSPG), tartaric acid, sodium tartrate, sodium carbonate, double distilled water, the specific dosage is shown in Table 2.
[0056] Table 2
[0057] Phosphate ester
DPPC
DSPE-mPEG2000
DSPG
Mass (g)
7.7
2
0.3
[0058] Weigh various phospholipids (the amount used to prepare 100ml blank liposomes) according to Table 2, and dissolve them in 80ml chloroform and methanol solution (1∶1) to form a clear and transparent solution. Rotary evaporation at 60°C to remove organic solvents to obtain a dry, uniform Film attached to the bottom of the glass bottle. Hydrate with (100ml, 0.2M, pH4) tartrate buffer to obtain a multilamellar liposome solution. Extrusion through a polycarbonate membrane, first through a polycarbonate membrane wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com