Method for the isolation of subpopulations of cardiac progenitor cells and related uses in the medical field
A technology of cell subsets and heart cells, which can be used in medical preparations containing active ingredients, animal cells, vertebrate cells, etc., and can solve problems such as affecting the background separation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097][Example 1: Method for Isolating Human Cardiac Progenitor Cell Population by Positive Selection]
[0098] 【Materials and methods】
[0099] 【Primary culture】
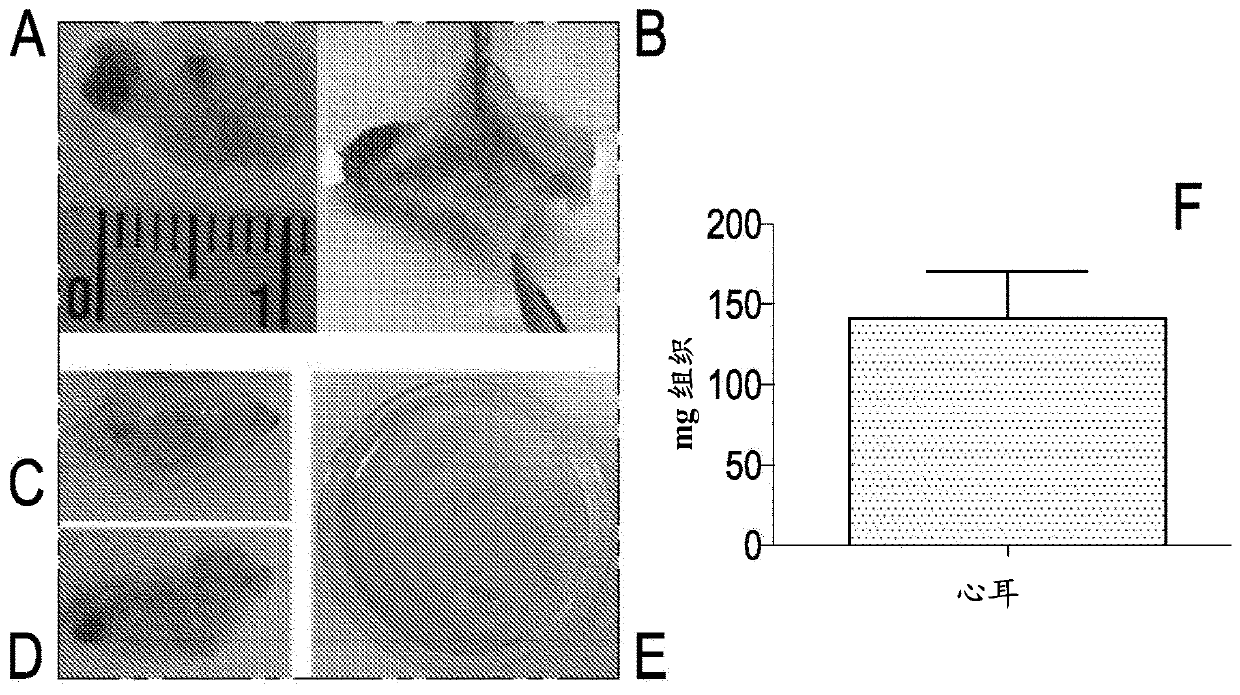
[0100] Samples of auricles weighing between 36.8 and 631.8 mg were collected in the operating room (see figure 1 ), and immediately transfer to a sterile solution containing at least one sterile solution (phosphate-buffered saline, PBS, or physiological solution), preferably one that also maintains tissue viability (such as any medium) to prevent its dehydration in the container.
[0101] Alternatively, another solution comprising bovine serum albumin (BSA) or human serum albumin (HSA) or fetal bovine serum (FBS) may also be used. From then on, samples can be kept at controlled temperature (+4 °C) and processed within 48 h, or frozen in a solution containing at least FBS and DMSO in liquid nitrogen, since freezing has no bearing on whether cultures are obtained impact (see Figure 6 a-b). For processing, aft...
Embodiment 2
[0135] [Example 2: Method for Isolating Human Cardiac Progenitor Cell Population by Negative Selection]
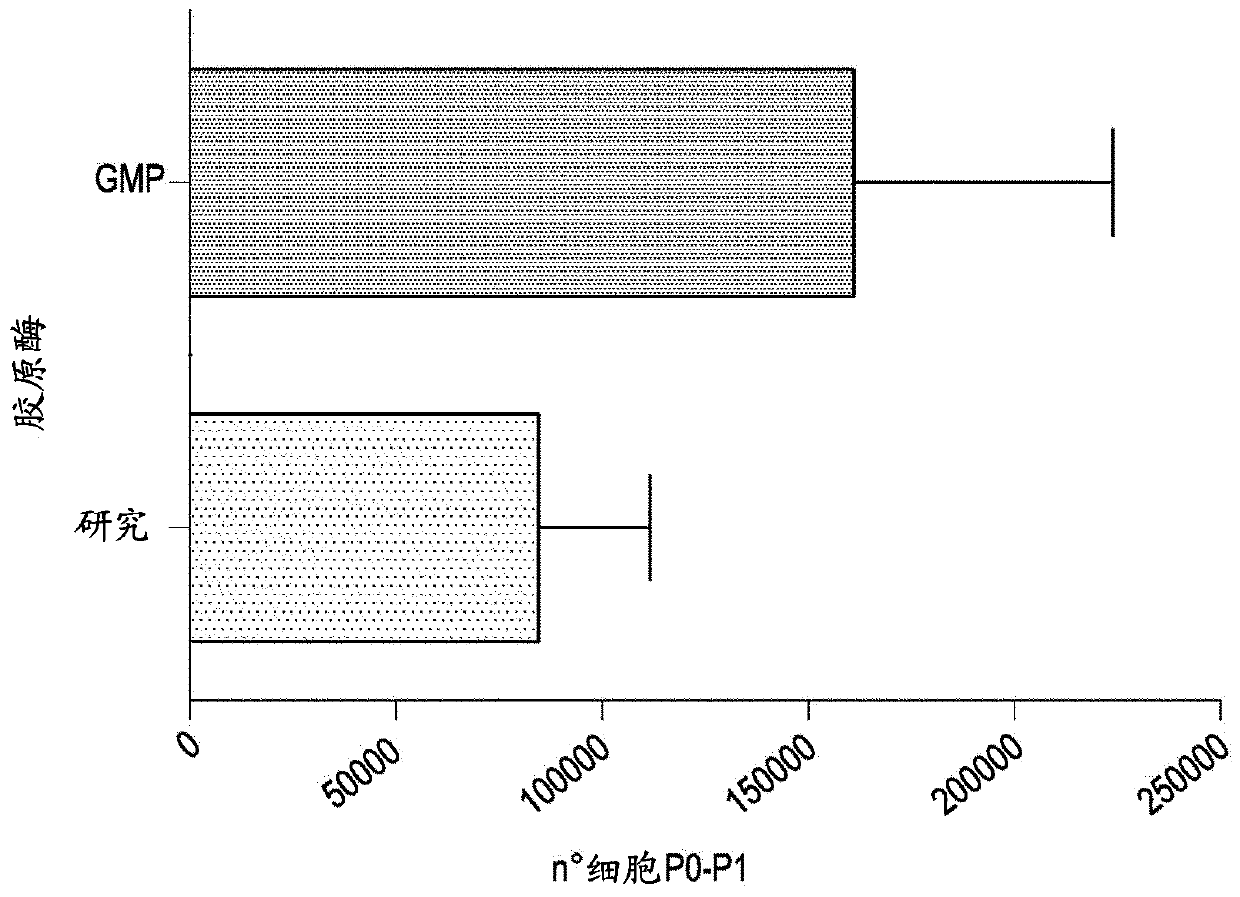
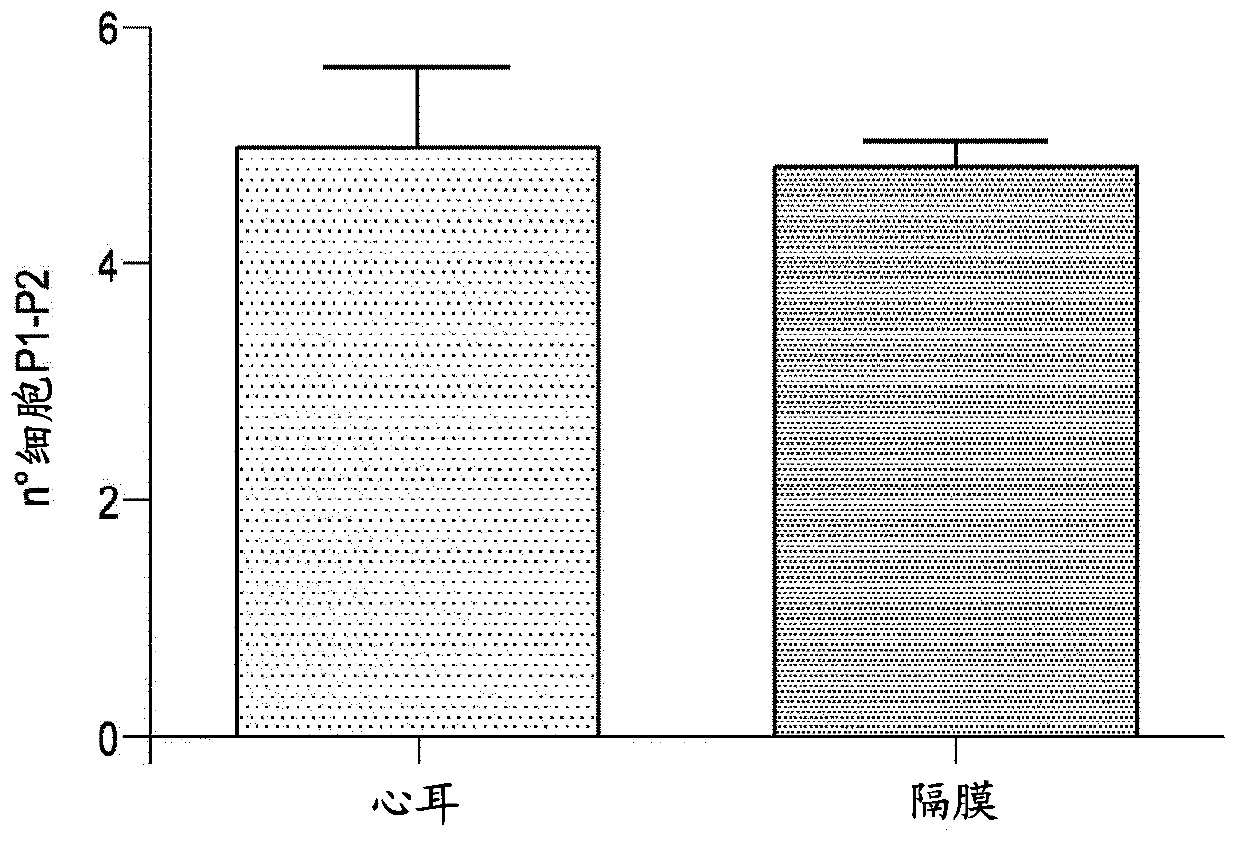
[0136] Before selection, the steps of fragment digestion, primary culture and amplification were the same as in Example 1.
[0137] 【Isolate a population of interest by negative selection】
[0138] Isolate the population to be isolated from the dish using a non-enzymatic method (see section "Expansion of non-selected populations"), count the cells and resuspend in cold isolation buffer (WB); 7 Resuspend the cells at a concentration of 100 μl.
[0139] Before negative selection, a fraction of cells (100,000) were used for fluorescence activated cell sorting (FACS) analysis. Cells are labeled with immunoglobulin (isotype IgG) conjugated with the same fluorescent molecule as the antibody (in this example FITC, BD) that recognizes the target population, 1 μl of antibody, and incubated at room temperature in Incubate for 15 minutes in the dark or with anti-biotin FITC (Milte...
Embodiment 3
[0150] [Example 3: Method for Isolating Human Cardiac Progenitor Cell Populations by Combined Positive and Negative Selection]
[0151] Before selection, the steps of fragment digestion, primary culture and amplification were the same as in Example 1.
[0152] [Isolate the population of interest by combining positive and negative selection]
[0153] Isolate the population to be isolated from the dish using a non-enzymatic method (see section "Expansion of non-selected populations"), count the cells and resuspend in cold isolation buffer (WB); 7 cells without resuspending the cells at a concentration of 100 μl.
[0154] Prior to selection, a fraction of the cells (400,000) were subjected to fluorescence-activated cell sorting (FACS) analysis ( Figure 11 A-E). Subsequently, the cells were divided into four different tubes.
[0155] The first tube (identified as isotype FITC) is labeled with an immunoglobulin (isotype IgG) that binds to the same fluorescent molecule that bin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com