Molecular sensor capable of singly and selectively identifying picric acid molecules and synthesis and application of molecular sensor
A technology of molecular sensor and picric acid, which is applied in the direction of chemical instruments and methods, instruments, analytical materials, etc., can solve the problems of easily polluted soil and groundwater, and the reduction of biological treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
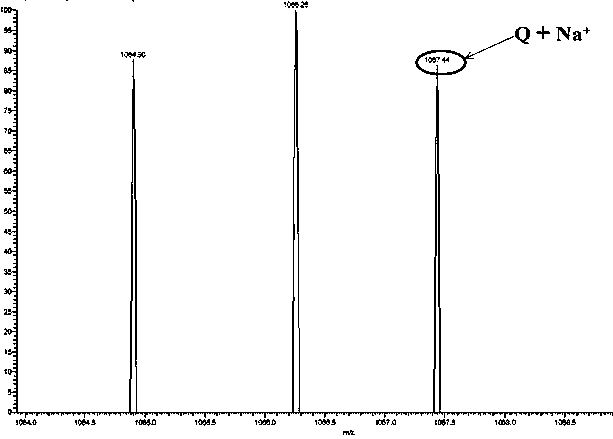
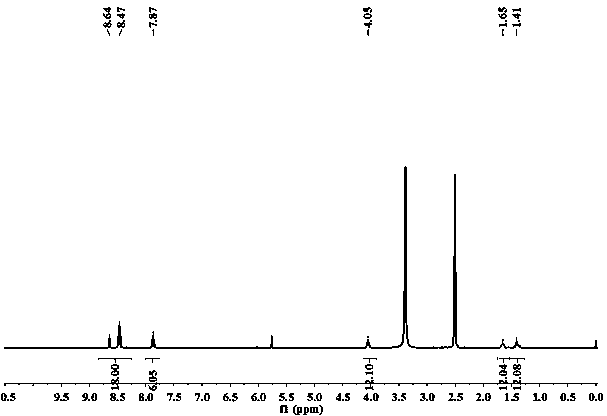
[0030] Embodiment 1, molecular sensor Q
[0031] Weigh 0.19 g (1 mol) of 1,8-naphthalene dioic anhydride and 0.345 g (3.0 mol) of hexamethylenediamine, add them into 60 ml of ethanol solvent, stir to dissolve completely, and react at 85°C for 36 h. After the reaction Cooling precipitated a white solid, suction filtered, and rinsed with ethanol to obtain 0.25 naphthalimide derivatives with a yield of 83%;
[0032] Weigh 1.18 g (4 mol) of naphthalimide derivatives, 0.26 g (1 mol) of trimesoyl chloride, add them to 65 ml of dichloromethane, and react at room temperature for 48 h; after the reaction, a white solid precipitates out and is suction filtered , rinsed with hot ethanol, the product obtained is the molecular sensor Q, and the yield is 63%.
Embodiment 2
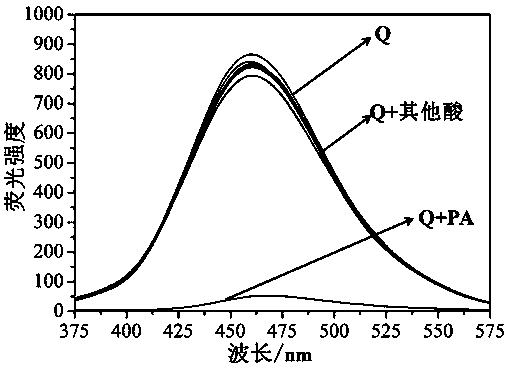
[0033] Example 2. Molecular sensor Q recognizes PA
[0034] Pipette 2 ml of supramolecular sensor molecule Q in DMF-H 2 O solution (C Q =1×10 -5 M, V DMF : V 水 = 1:9) In a series of colorimetric tubes, add the DMF solutions of picric acid, hydrochloric acid, acetic acid, n-butyric acid, salicylic acid, hippuric acid, phenylhydrazine-4-sulfonic acid and p-hydroxybenzoic acid respectively (C =0.01M), if the DMF-H of the sensor molecule 2 The fluorescence of the O solution is obviously quenched, which means that the solution of picric acid molecules is added, and if the fluorescence intensity of the sensor molecules does not change, it means that the solution of picric acid molecules is not added.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com