Fused ring aromatic imide derivative compound, preparation method and applications thereof
A technology of fused-ring aromatic imides and derivatives, which is applied in the field of fused-ring aromatic imide derivatives and their preparation, can solve the problems of simple coordination form, single coordination point, single structure and function, etc. Achieve the effect of simple purification, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
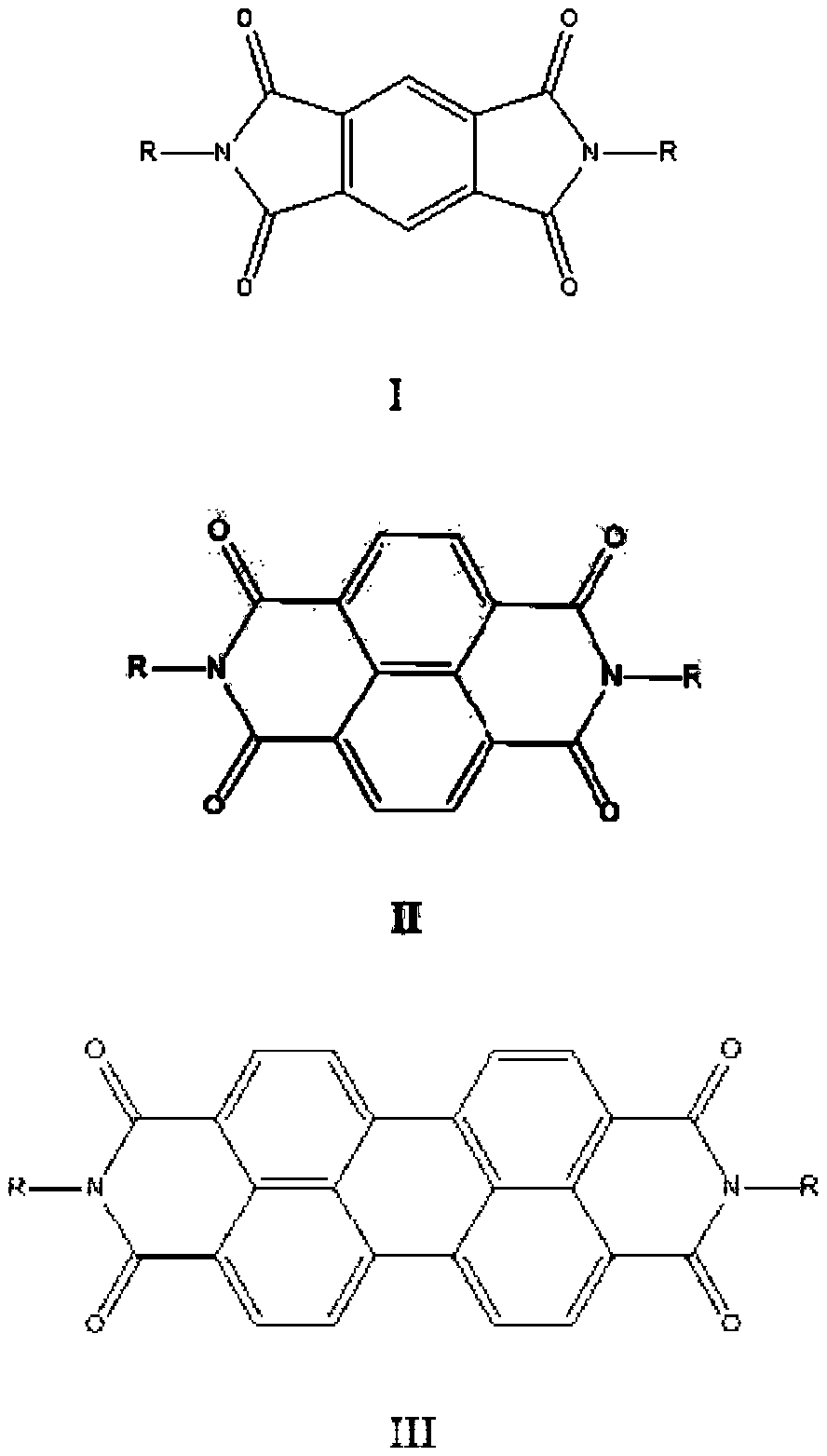
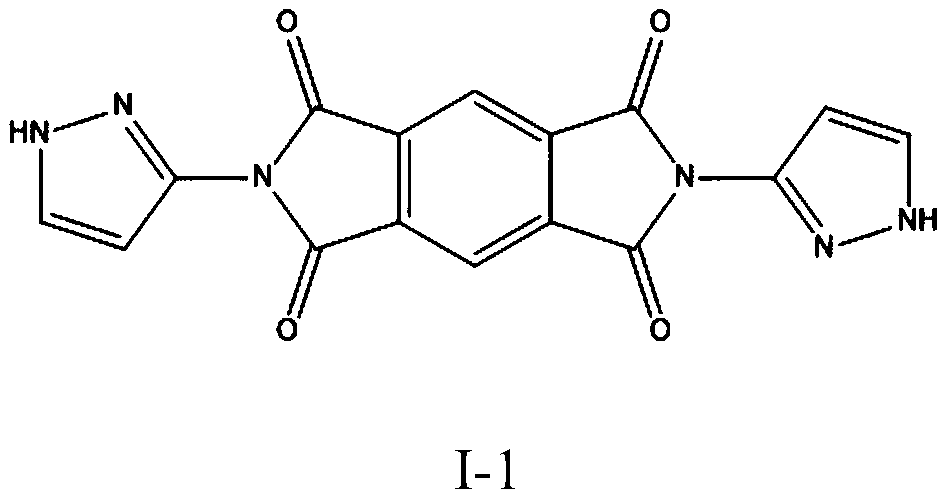
[0034] This embodiment provides a fused-ring aromatic imide derivative compound, the structural formula of which is:
[0035]
[0036] The synthetic route for preparing compound I-1 is as follows:
[0037]
[0038] Specific steps are as follows:
[0039] Under nitrogen protection, pyromellitic anhydride (4.58mmol) was refluxed in anhydrous N,N-dimethylacetamide (50mL) for 5min; 3-aminopyrazole (9.16mmol) was dissolved in anhydrous N,N -Dimethylacetamide (5mL) was then injected into the reaction flask with a syringe; the mixture was stirred and refluxed for 6h; the reaction was completed, cooled to room temperature, filtered with suction, the filter cake was washed with dichloromethane, and dried in vacuo to obtain compound I-1, Yield 91%;
Embodiment 2
[0041] This embodiment provides a fused-ring aromatic imide derivative compound, the structural formula of which is:
[0042]
[0043] The synthetic method for preparing compound I-2 is the same as that of Example 1, except that 3-aminopyrazole is replaced by 5-methyl-3-aminopyrazole; the yield of compound I-2 is 89%.
Embodiment 3
[0045] This embodiment provides a fused-ring aromatic imide derivative compound, the structural formula of which is:
[0046]
[0047] The synthetic route for preparing compound II-1 is as follows:
[0048]
[0049] Specific steps are as follows:
[0050] Under nitrogen protection, 1,4,5,8-naphthalene tetracarboxylic anhydride (3.2mmol), 3-aminopyrazole (6.8mmol) were refluxed in anhydrous N,N-dimethylformamide (50mL) for 9h; After the reaction was completed, cool to room temperature, pour the solution into diethyl ether (150mL), filter with suction, recrystallize the filter cake with N,N-dimethylformamide / diethyl ether (20mL / 30mL), filter and dry in vacuo to obtain compound II- 1. The yield is 82%;
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap