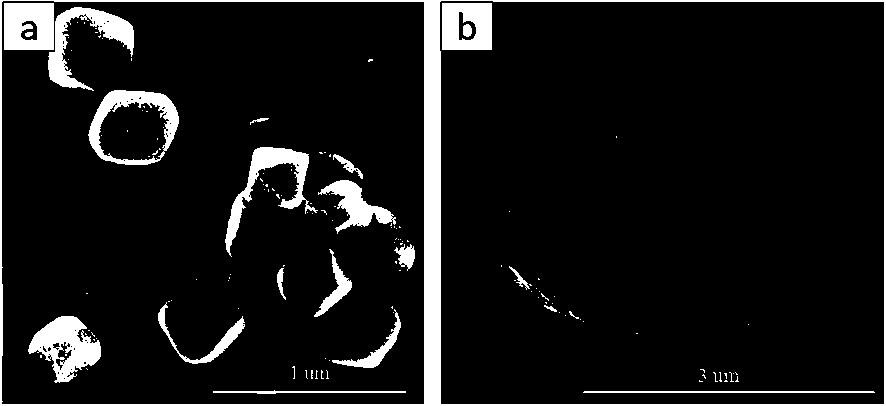
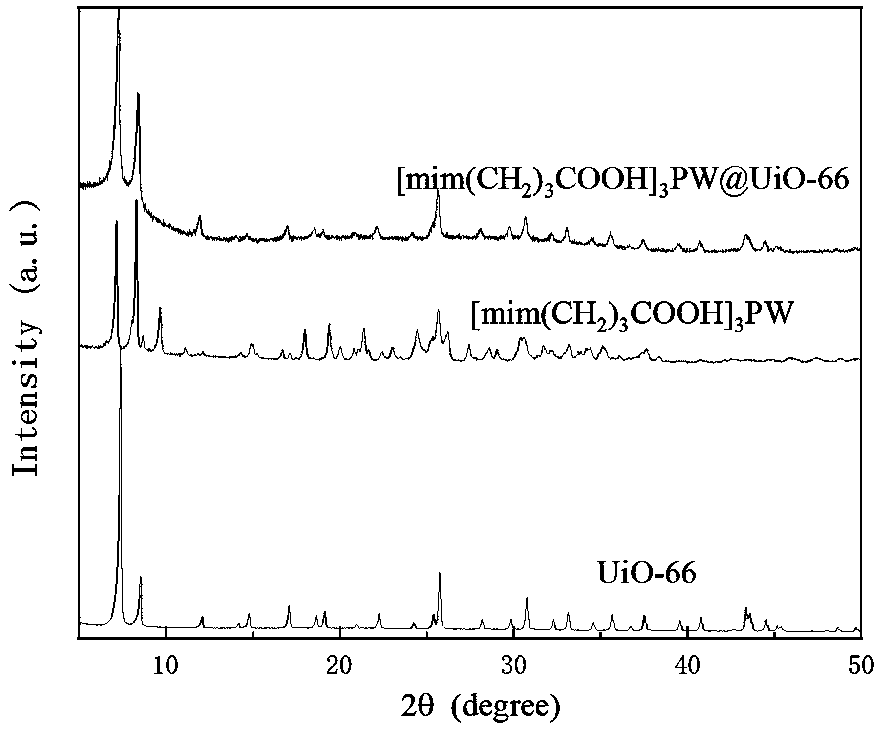
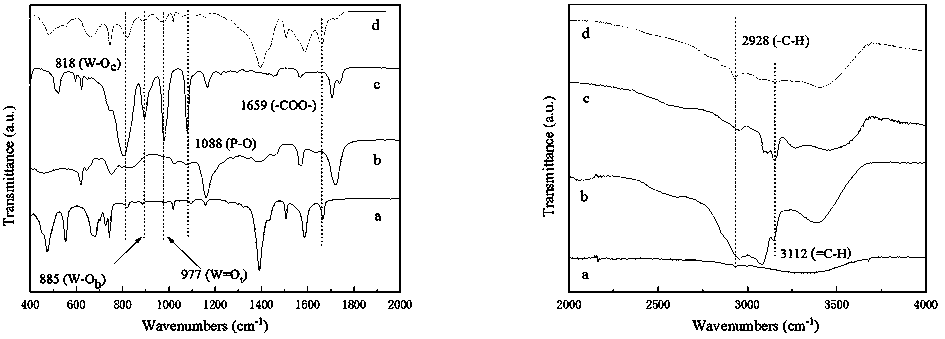
Method for in-situ bridging packaging of heteropoly acid ionic liquid by Zr-based MOFs
A technology of ionic liquids and heteropolyacids, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, hydrocarbon oil treatment, etc. Catalytic activity and other issues, to achieve the effect of good effect, excellent catalytic performance and reusable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) [mim(CH 2 ) 3 COOH] 3 Preparation of PWs
[0026] Accurately weigh 0.01 mol of methyl 4-bromobutyrate in a 25 mL round bottom flask, add 0.01 mol of 1-methylimidazole (molar ratio 1:1) dropwise under magnetic stirring, and reflux at 80 °C After 48 h, a light yellow viscous substance was obtained, which was cooled to room temperature, washed with ether (10 mL×3), and dried in vacuo. The resulting product was added dropwise with 0.01 mol HCl (40% aqueous solution) under magnetic stirring. Acidify, reflux at 80°C for 30 min, cool to room temperature, wash with ether (10 mL×3), and dry in vacuo to obtain 1-carboxypropyl-3-methylimidazolium chloride ([mim(CH 2 ) 3 COOH]Cl). [mim(CH 2 ) 3 Dissolve COOH]Cl in 50 mL deionized water to make solution A, then weigh 0.002 mol of phosphotungstic acid (HPW) and dissolve it in 40 mL deionized water to make solution B, add solution A to solution B dropwise , stirred at room temperature for 12 h, filtered and washed, the res...
Embodiment 2
[0034] Taking dibenzothiophene as the target sulfide, it was dissolved in n-octane to prepare simulated gasoline with a concentration of 1000 ppmS. Take 5 mL of the above simulated gasoline, 0.04 g [mim(CH 2 ) 3 COOH] 3 PW@UiO-66 and 5 mL of acetonitrile were added to a 25 mL round bottom flask, stirred at room temperature for 10 min, and then 0.106 g of H 2 o 2 (30% aqueous solution), reacted at 60 °C for 2 h, cooled to room temperature, and analyzed the upper oil phase by gas chromatography to detect the content of dibenzothiophene. It was determined that under the above reaction conditions, [mim(CH 2 ) 3 COOH] 3 The oxidation removal rate of PW@UiO-66 for dibenzothiophene can reach 100%.
Embodiment 3
[0036] Taking benzothiophene as the target sulfide, it was dissolved in n-octane to prepare simulated gasoline with a concentration of 1000 ppmS. Take 5 mL of the above simulated gasoline, 0.04 g [mim(CH 2 ) 3 COOH] 3 PW@UiO-66 and 5 mL of acetonitrile were added to a 25 mL round bottom flask, stirred at room temperature for 10 min, and then 0.106 g of H 2 o 2 (30% aqueous solution), reacted at 60 °C for 2 h, cooled to room temperature, and analyzed the upper oil phase by gas chromatography to detect the content of benzothiophene. It was determined that under the above reaction conditions, [mim(CH 2 ) 3 COOH] 3 The oxidation removal rate of PW@UiO-66 for thiophene can reach 95.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com