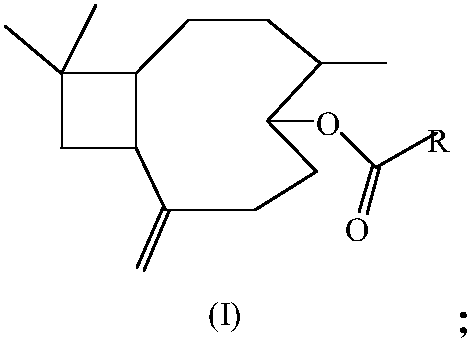
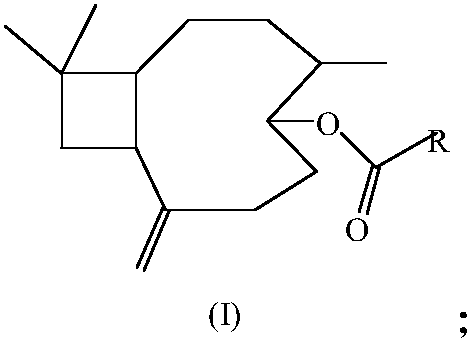
Pyrazinecarboxylic acid beta-caryophyllene-5-ester compound as well as preparation method and application thereof
An ester compound, pyrazinecarboxylic acid technology, applied in the directions of organic chemistry, drug combination, antipyretics, etc., can solve the problems such as no reports, and achieve the effects of good inhibitory activity, simple reaction steps, and good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
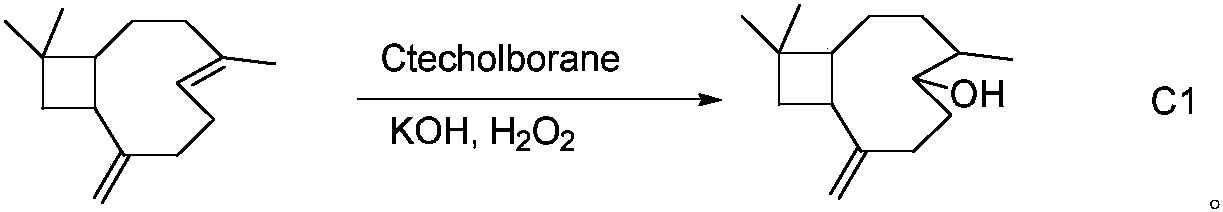
[0031] The synthesis method of β-caryophyllene alcohol C1 (6,10,10-trimethyl-2-methylenebicyclo[7.2.0]undecan-5-ol):
[0032] Add 4.4mmol of β-caryophyllene to 6.6mL of 1M solution of catecholborane in tetrahydrofuran, reflux at 80°C for 18h, and use 40mL of CH 2 Cl 2 Dilute and cool, add 20mL 3M KOH and 20mL 30% H 2 o 2 , reacted for 30 min, washed 3 times with saturated NaCl, dried, and desolventized to obtain a dark yellow oily liquid, which was eluted with a 100-200 mesh silica gel column, mobile phase petroleum ether: ethyl acetate=1: 7, and a light yellow oily liquid was obtained C1. The reaction process is as follows:
[0033]
[0034] C1 1 HNMR (600M, DMSO-d 6 ) δ: 4.83 (d, 2H, =CH 2 , J=6Hz), 4.22(d, 1H, -OH, J=6Hz), 3.32(s, 1H, -CH), 2.46-2.42(m, 1H, -CH), 2.22-2.12(m, 2H, -CH 2 ), 1.92-1.86 (m, 1H, -CH), 1.74-1.71 (m, 2H, -CH 2 ), 1.70 (t, 1H, -CH, J=6Hz), 1.58-1.53 (m, 1H, -CH), 1.51-1.48 (m, 2H, -CH 2 ), 1.44-1.39 (m, 2H, -CH 2 ), 0.38(s, 3H, -CH ...
Embodiment 2
[0041] 1. NO inhibition rate experiment of compound C2:
[0042] (1) Take the mouse macrophage RAW264.7 with a logarithmic growth cycle, seed it in a 96-well plate at 30,000 to 40,000 per well, at 37°C, 5% CO 2 Incubate in an incubator for 24 hours; take out the culture plate, remove the medium, and wash with PBS for 3 to 4 times;
[0043] (2) Set up the control group, LPS+dexamethasone (DIM) positive drug group and compound C2 sample group; the experimental groups are as follows:
[0044] Control group: 1. Add 50 μL of 2 μg / mL LPS and 50 μL of caryophyllene CO at concentrations of 40, 20, 10, 5, and 2.5 μM to each well; 2. Add 50 μL of 2 μg / mL LPS and 50 μL of LPS to each well β-caryophyllenol C1 at 40, 20, 10, 5, 2.5 μM, respectively;
[0045] LPS+DIM positive drug group: Add 50 μL of 2 μg / mL LPS and 50 μL of DIM with concentrations of 40, 20, 10, 5 and 2.5 μM to each well;
[0046] Compound C2 sample group: add 50 μL of 2 μg / mL LPS and 50 μL of C2 at concentrations of 40...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com