Preparation method of ozagrel impurity
A technology of impurity and mass ratio, applied in the field of preparation of ozagrel impurities, can solve the problems of appearance variation, activity reduction, low drug content and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] Further description will be made below in conjunction with the accompanying drawings and specific embodiments.
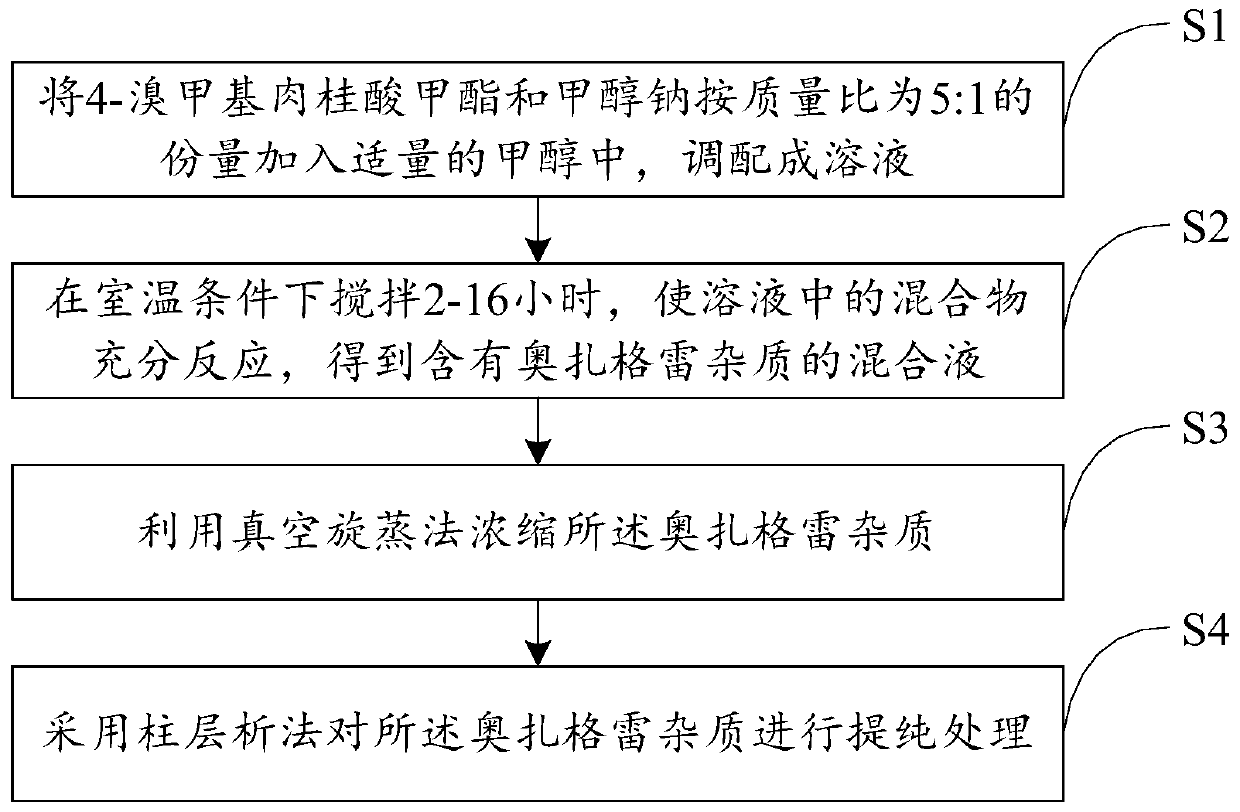
[0021] Such as figure 1 and figure 2 As shown, the present invention provides a kind of preparation method of ozagrel impurity, comprises the following steps:
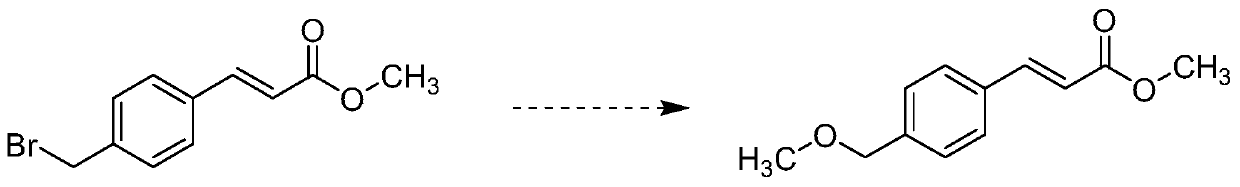
[0022] S1: adding methyl 4-bromomethyl cinnamate and sodium methoxide in a mass ratio of 5:1 to an appropriate amount of methanol to prepare a solution;
[0023] S2: stirring at room temperature for 2-16 hours to fully react the mixture in the solution to obtain a mixed solution containing ozagrel impurities;
[0024] S3: Concentrating the ozagrel impurity by vacuum rotary evaporation;
[0025] S4: Purify the ozagrel impurity by column chromatography.
[0026] For making 4-bromomethyl cinnamic acid methyl ester dissolve completely, the content of methyl alcohol is as many as possible, present embodiment adds 1 part of 4-bromomethyl cinnamic acid methyl ester and 0.2 part of sodium methylate in 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com