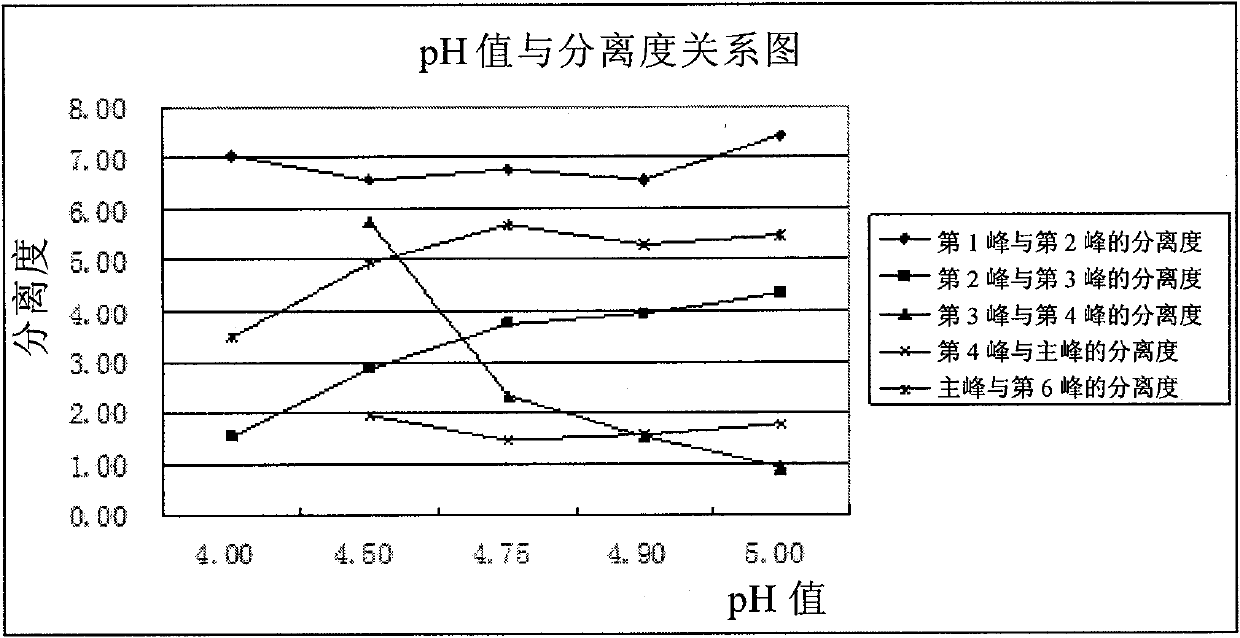
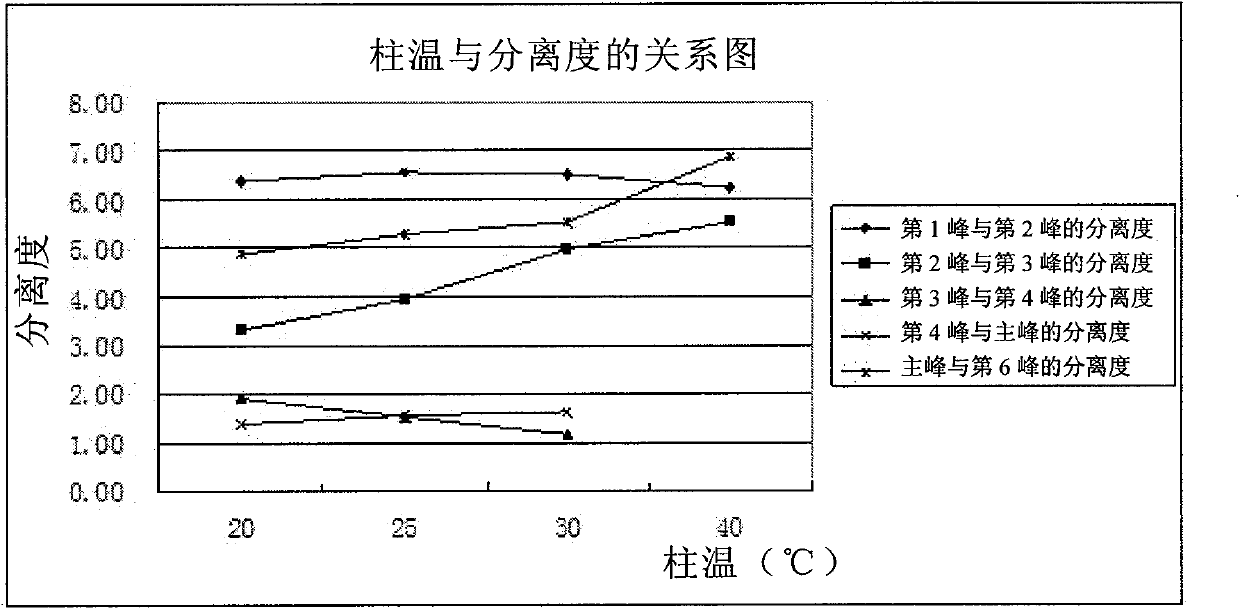
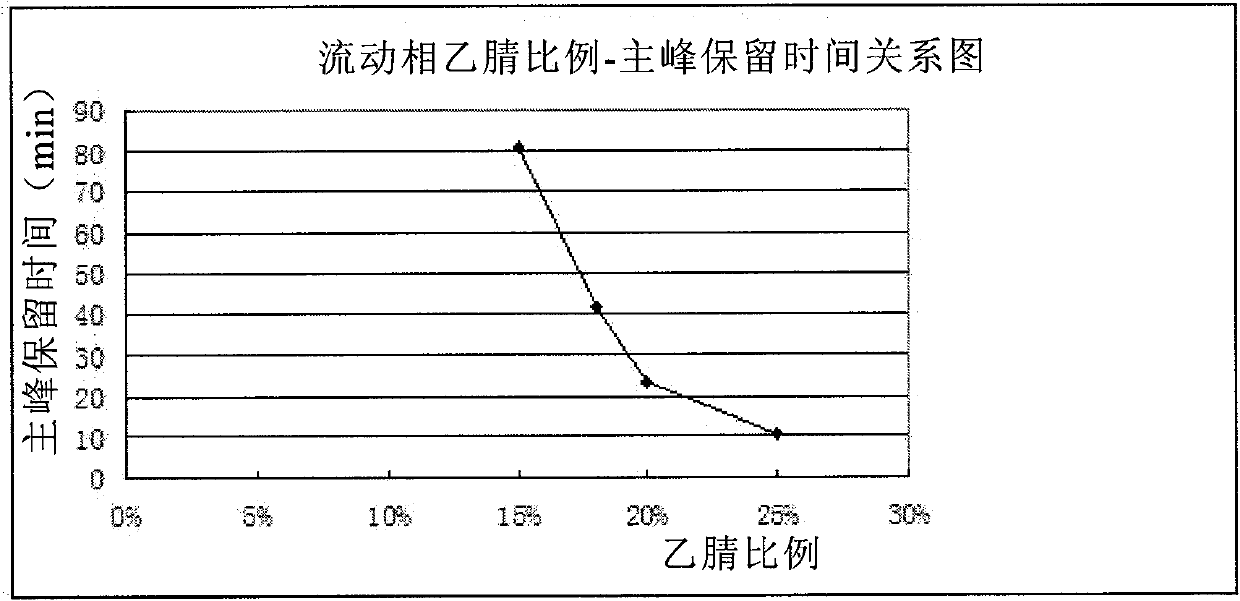
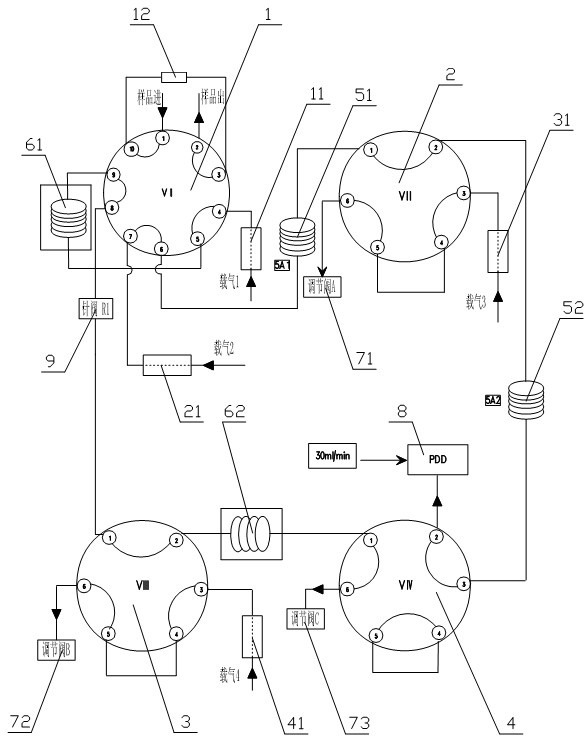
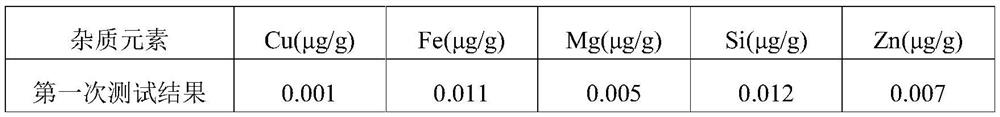
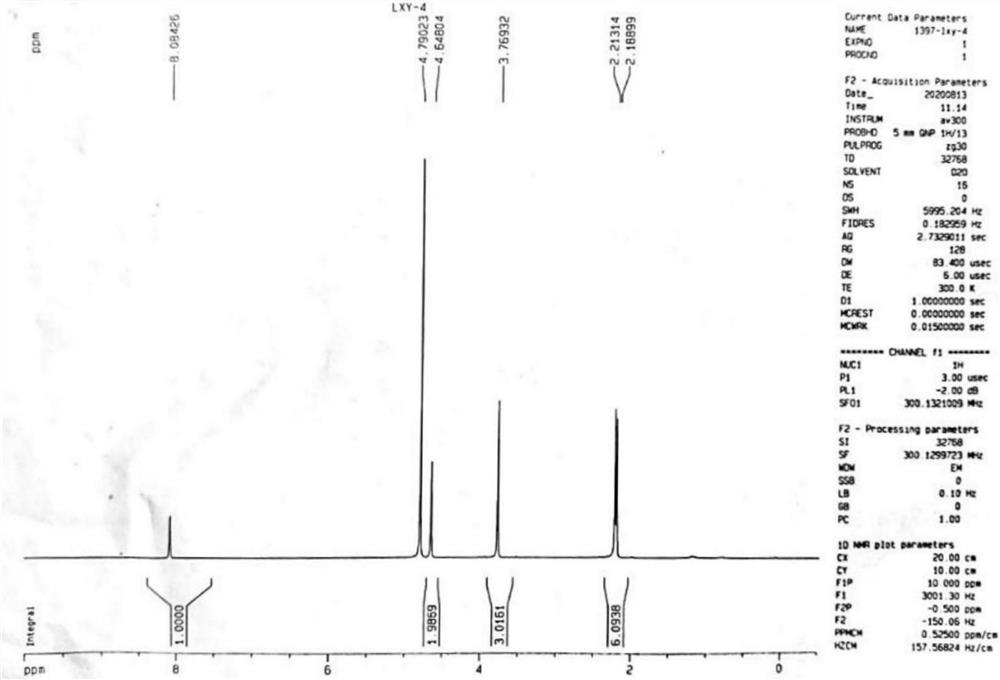
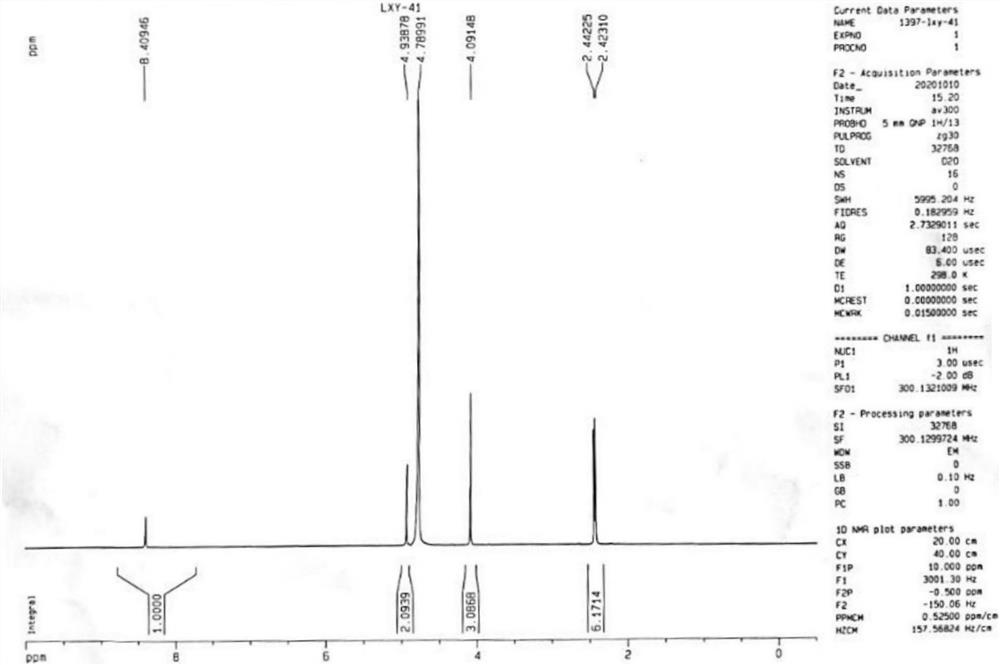
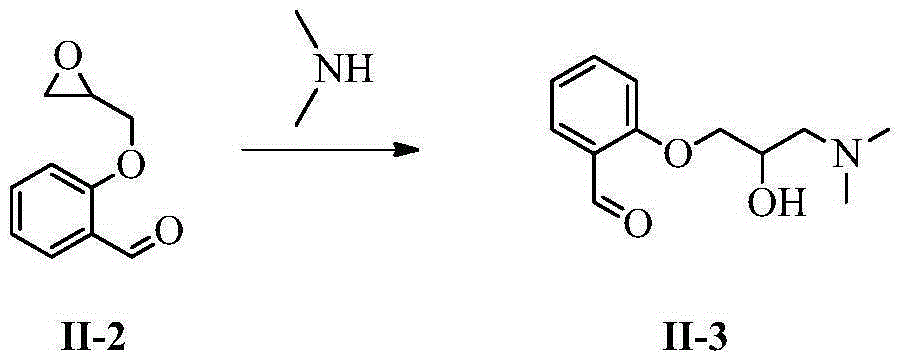
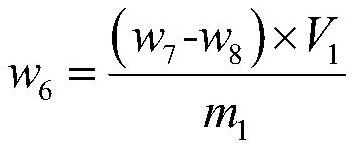Patents
Literature
31 results about "Impurity profiling" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
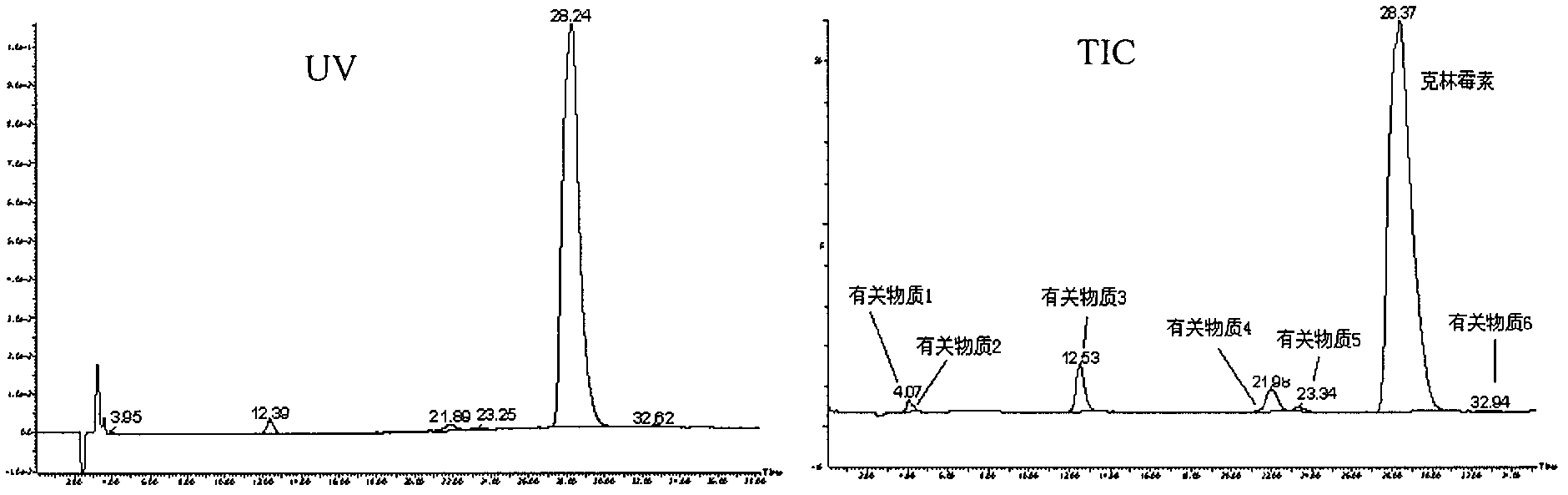
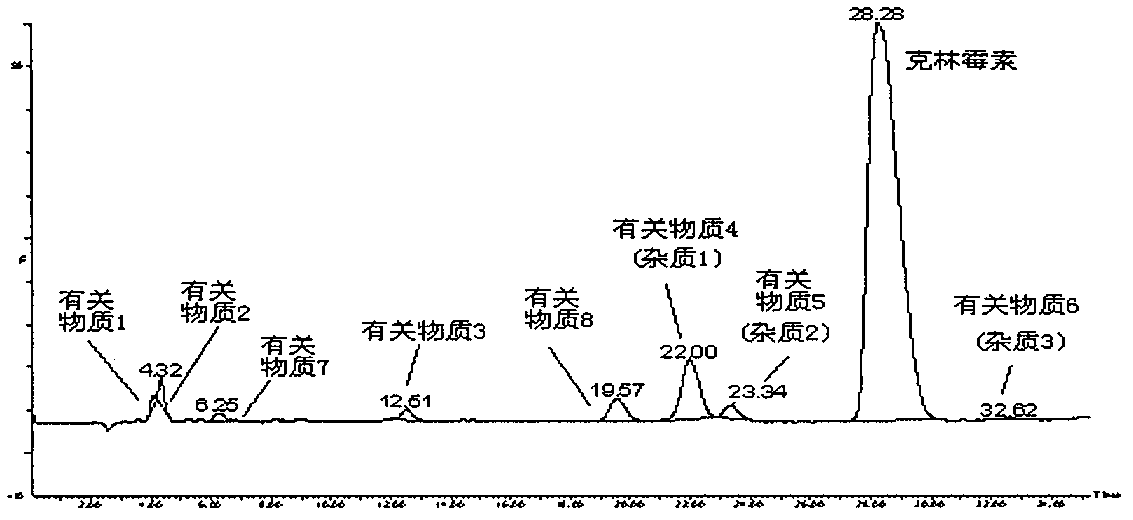
Impurity analysis and preparation method for clindamycin phosphate
The invention provides an impurity analysis and preparation method for clindamycin phosphate, which is used for analyzing a clindamycin phosphate raw material and separating and preparing impurities from the clindamycin phosphate raw material. The method comprises the following steps of: measuring the clindamycin phosphate raw material by using liquid chromatography-mass spectrometry (LC-MS), and determining one or more impurities in the raw material according to the relative retention time and / or molecular weight of each analyzed component; and determining the conditions of preparative chromatography according to chromatographic retention behaviors displayed by the relative retention time of each impurity, and collecting the one or more impurities corresponding to the relative retention time and / or molecular weights by using the preparative chromatography.
Owner:浙江天台药业股份有限公司
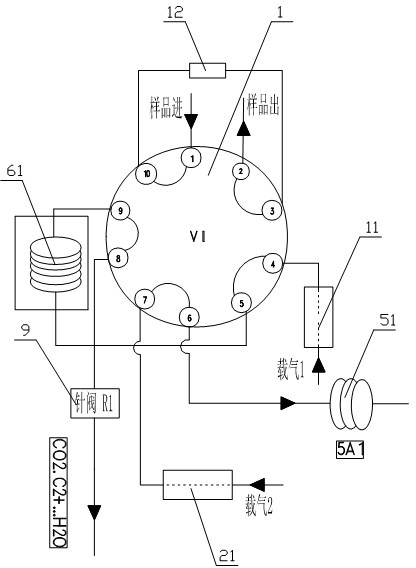
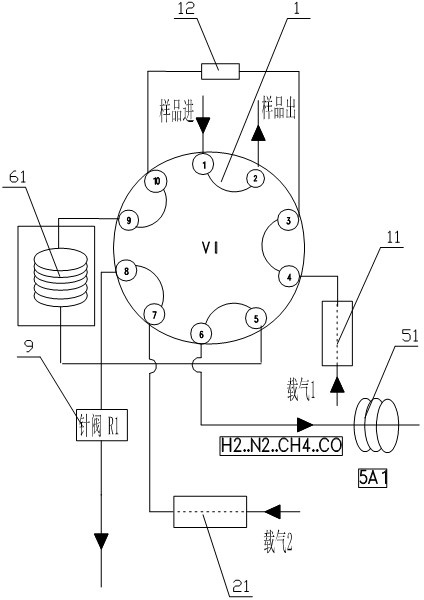
Gas chromatography detection system and method for analyzing trace impurities in ultrahigh pure gas
ActiveCN102628846AFast, consistent and reliable actionEnsure data accuracyComponent separationMolecular sieveGas liquid chromatographic
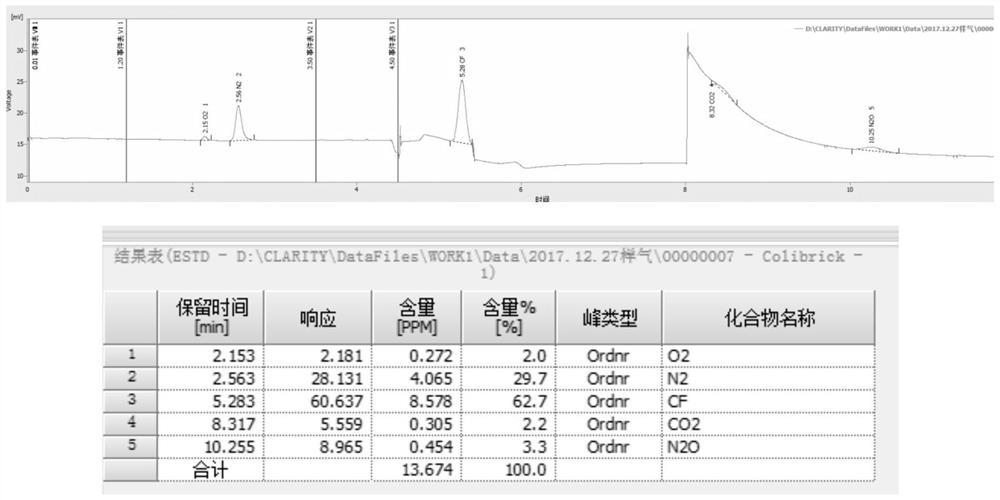
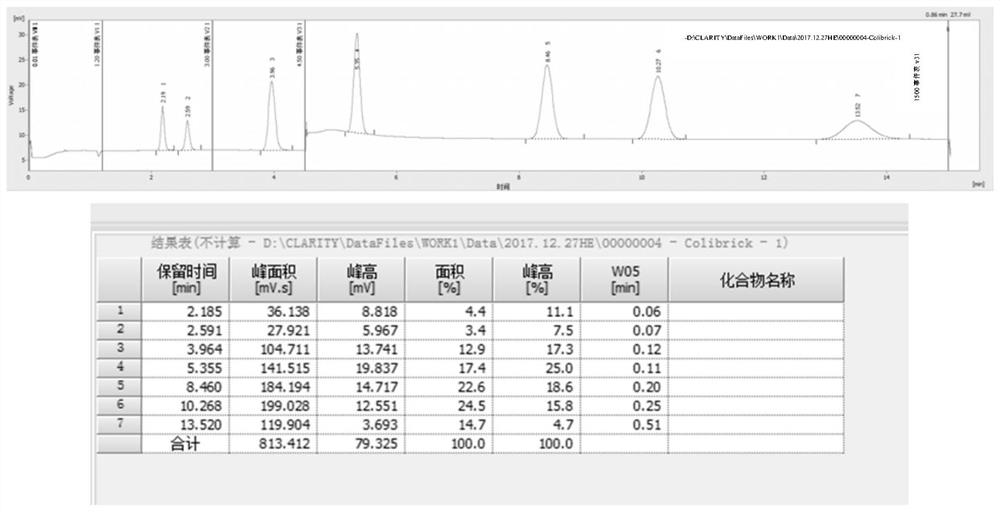
The invention relates to detection equipment and technology for a gas chromatography instrument, in particular to a gas chromatography detection system and method for analyzing trace impurities in ultrahigh pure gas. A first molecular sieve chromatographic column (51) is arranged between a switching valve VI (1) and a switching valve VII (2); a second molecular sieve chromatographic column (52) is arranged between a switching valve VII (2) and a switching valve VIV (4); and a second column separator (62) is arranged between the switching valve VIII (3) and switching valve VIV (4). A secondary sampling way is adopted for sample gas, and a main component is pre-cut during primary sample feeding of a ten-way switching valve VI (1) and subjected to back flushing during secondary sample feeding of the ten-way switching valve VI (1); and the main component is separated and switched, and enters a helium ion detector (8) for analyzing. The gas chromatography detecting system is controlled through each valve, the sequence of actions is executed by using an event draw-up program, the entire analyzing process is controlled automatically, actions are rapid, consistent and reliable, and the data repeatability and accuracy of the system are ensured through a stable flow gas channel and accurate valve switching.
Owner:HANGZHOU CHROMATOGRAPHY TECH
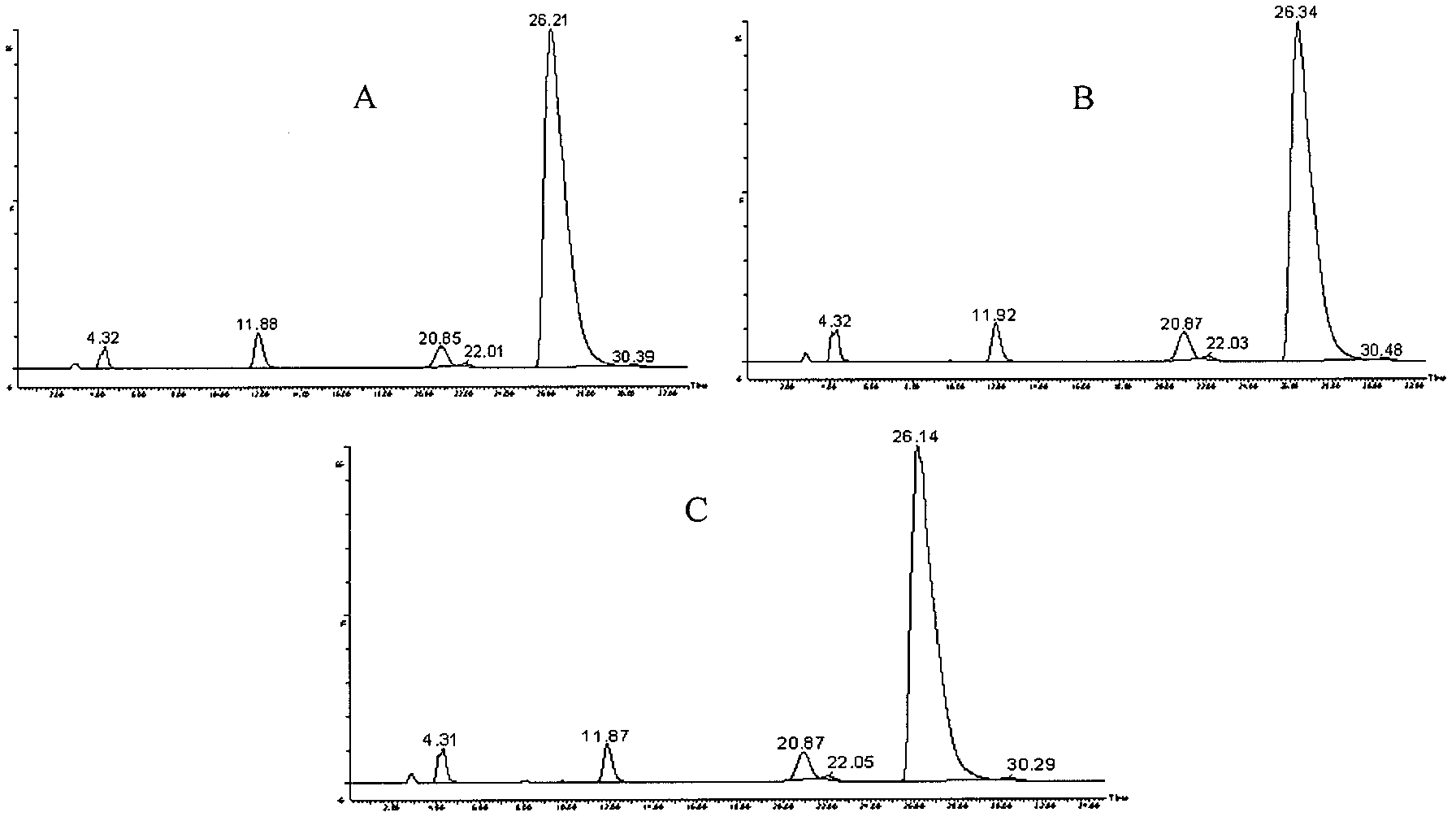
Impurity analysis preparation method for clindamycin
The invention provides an impurity analysis preparation method for clindamycin. The method is used for analyzing clindamycin materials and separating and preparing impurities from the materials, and comprises the following steps of: (a) measuring the clindamycin materials by an LC-MS (Liquid Chromatography-Mass Spectrometry) method, and determining one or more impurities in the materials according to the relative retention time and / or the molecular weight of the analyzed component; (b) determining the conditions of a column chromatography method according to the relative retention time and / or the molecular weight of one or more impurities, and gathering one or more impurities corresponding to the relative retention time and / or the molecular weight by the normal phase silica gel column chromatography method; and (c) determining the conditions of a preparative chromatography method according to the chromatographic retention behavior displayed by the relative retention time of one or more impurities in the step (a), and collecting one or more impurities corresponding to the retention time by the preparative chromatography method.
Owner:浙江天台药业股份有限公司
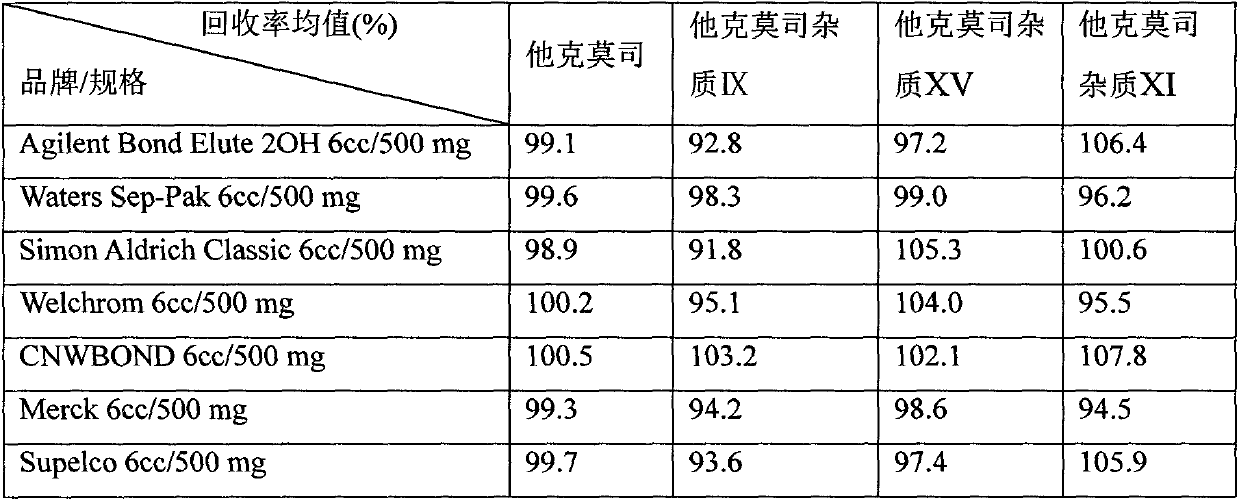
Application of solid-phase extraction column in analysis of tacrolimus preparation impurities
InactiveCN109828038APrecise control of impuritiesControl securityComponent separationOrganic solventSilanes
The invention relates to an application of a solid-phase extraction column in analysis of tacrolimus preparation ointment. In the preparation process of the test sample, the solid-phase extraction column with diol silane bonded silica gel as a filler is adopted for purification, and through the steps of suspension, activating, sample loading, leaching and elution, the tacrolimus and related substances thereof in the tacrolimus ointment sample are effectively separated. Moreover, the organic solvent is saved, the time consumption is short, the specificity is good, auxiliary materials do not interfere with tacrolimus impurity detection, and the impurity recovery rate also meets the requirements. The method is particularly suitable for effectively separating tacrolimus and related substancesfrom a complex matrix of tacrolimus ointment.
Owner:GUANGZHOU MEDCAN PHARMATECH
Impurity detection analysis method of norethisterone derivatives and intermediates thereof
ActiveCN105277633AEasy to operateEfficient separationComponent separationWater methanolGradient elution
The invention relates to an impurity analysis method of a steroid compound, and especially relates to an impurity detection analysis method of norethisterone derivatives and intermediates thereof. The method comprises the following steps: (1) a chromatographic column with octadecylsilane chemically bonded silica as a filler is selected; a mobile phase gradient elution is carried out, and the mobile phase contains water, methanol and acetonitrile; the detection wavelength is 230-254nm; and (2) a proper amount of the test sample norethisterone derivatives or intermediates thereof are selected, methanol is precisely weighed for dissolving and diluting the test sample to form a sample solution of a certain concentration, the solution is uniformly shaken, and a high performance liquid chromatography analysis is carried out at a proper flow velocity and a proper column temperature, in order to record a chromatogram. The method can be used for rapidly and accurately realizing impurity analysis of norethisterone derivatives, and is especially suitable for the impurity analysis of norethisterone enanthate, and simultaneously can be used for impurity analysis of norethisterone derivative intermediates, and can be used for effectively tracing impurities generated in the synthesis process.
Owner:ZHEJIANG XIANJU PHARMA
Ketorolac impurity C and preparation method and application thereof
PendingCN112898307AHigh yieldSpecific responseOrganic chemistryTesting medicinal preparationsKetorolacPhysical chemistry
The invention discloses a ketorolac impurity C and a preparation method and application thereof. According to the method, the ketorolac impurity C is prepared by taking pyrrole as an initial raw material through a series of reactions such as substitution, oxidation and alkaline hydrolysis, and the ketorolac impurity C is one of important impurities of ketorolac drugs or preparations thereof and can be used for toxicological and pharmacological researches such as in-vivo absorption and metabolism of ketorolac, and can also be applied to research on stability and quality control of ketorolac preparations. The preparation method of the ketorolac impurity C has the advantages of simplicity in operation, safety in reaction and high purity and yield, and can be widely applied to the fields of impurity analysis, toxicological research, safety detection, stability judgment and the like of ketorolac bulk drugs and preparations thereof.
Owner:THE KEY LAB OF CHEM FOR NATURAL PROD OF GUIZHOU PROVINCE & CHINESE ACADEMY OF SCI
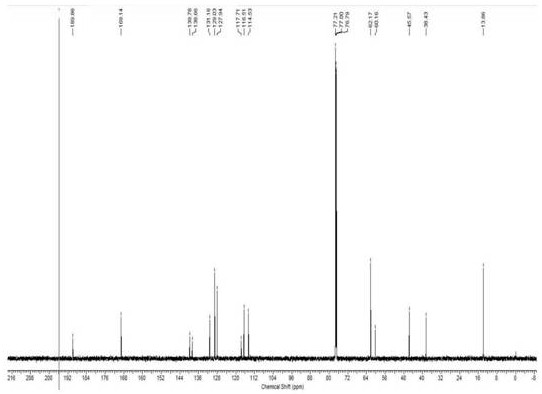
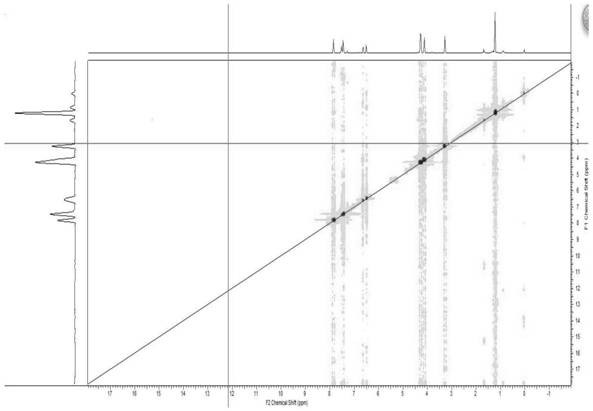
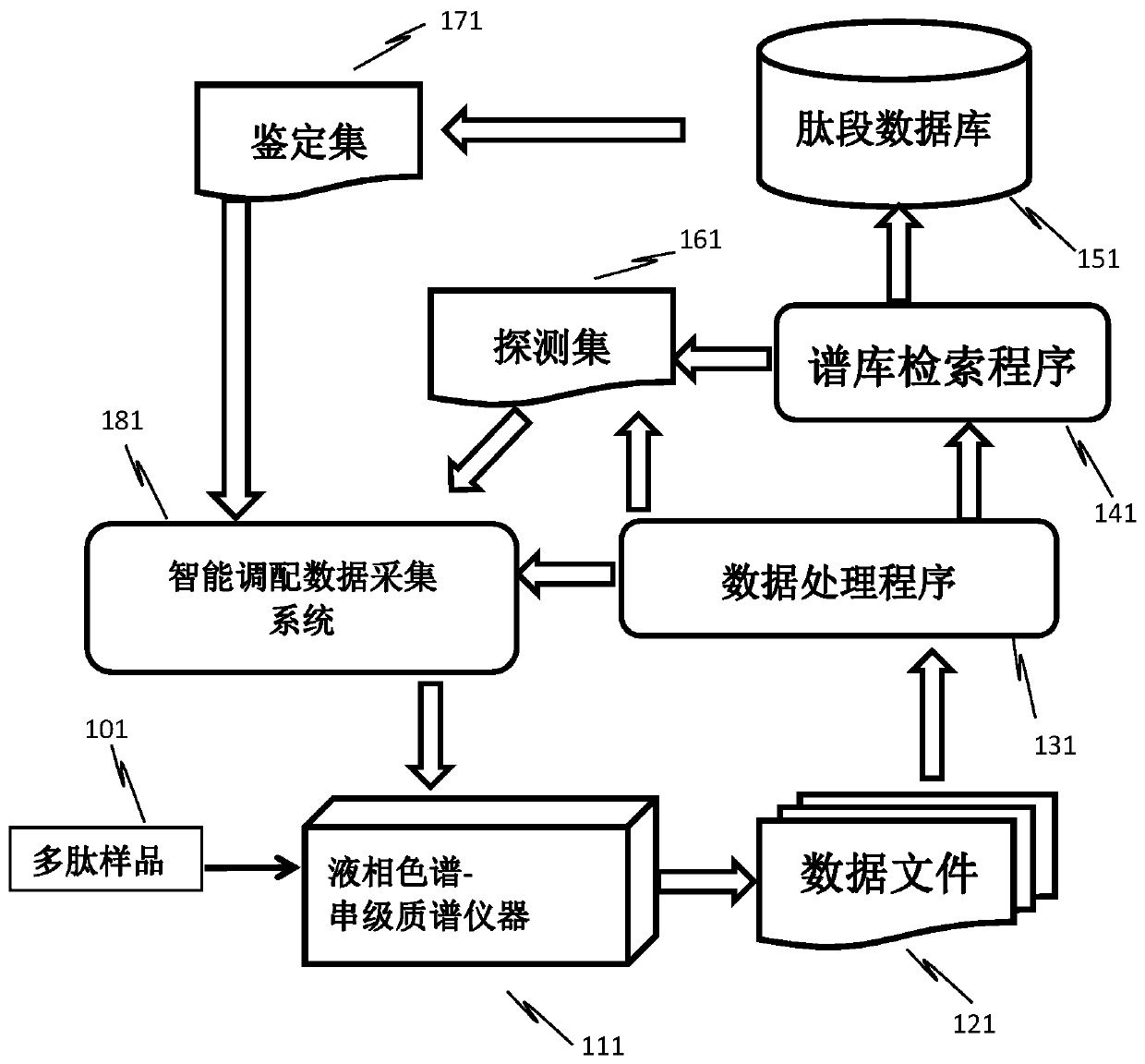
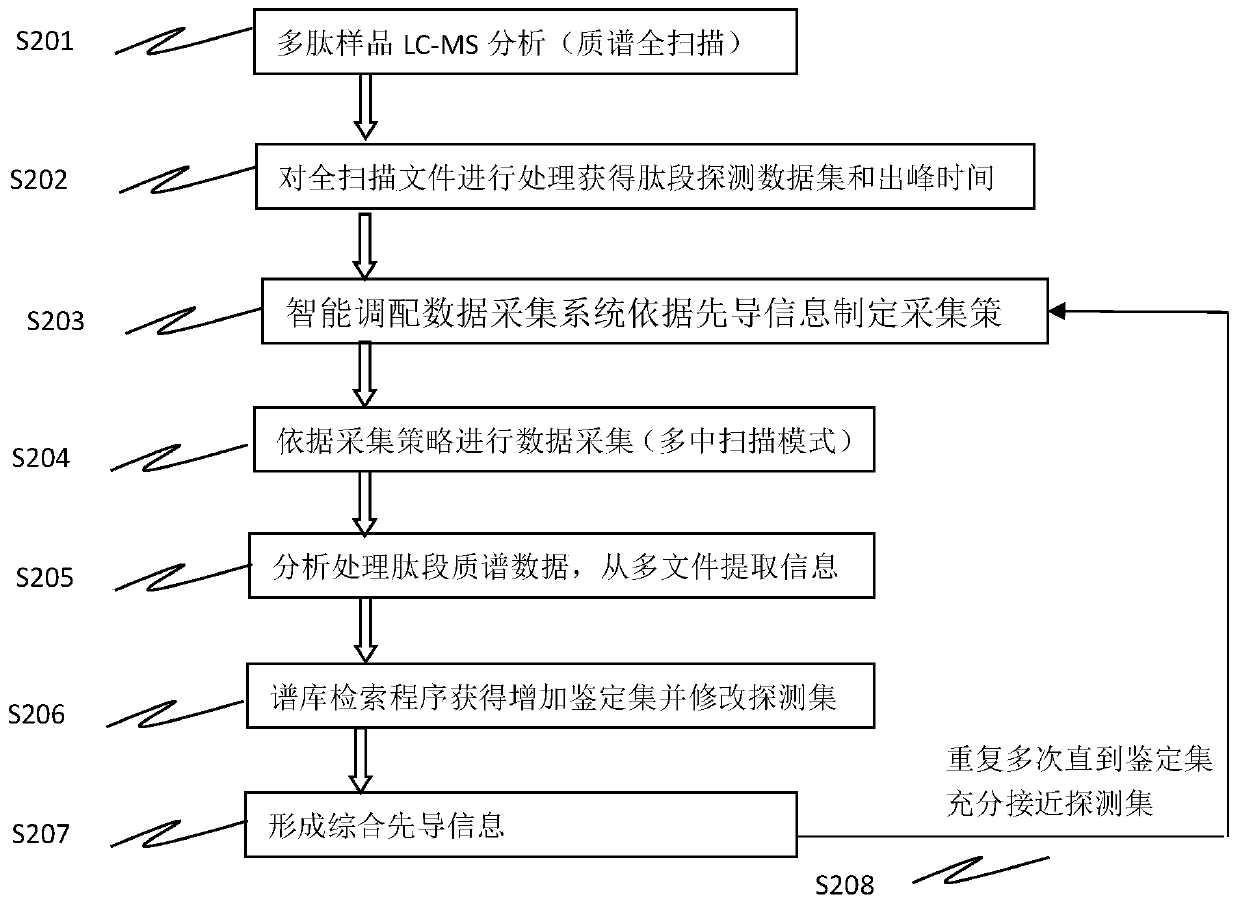
Method for analyzing peptide fragment impurities in high-purity polypeptide based on data mining
The invention provides a method for analyzing peptide fragment impurities in high-purity polypeptide based on data mining. The operation method for analyzing the peptide fragment impurities in the high-purity polypeptide comprises the following steps: during peptide fragment impurity analysis, based on a data dependence acquisition strategy, optimizing a detection data set and an identification data set while analyzing; adopting an onion peeling strategy to sequentially identify high-abundance impurity peptide fragments, wherein the subsequent analysis is no longer specific to the identified peptide fragments, the precious window period is saved, and the non-identified second-high-abundance peptide fragment impurities are identified; and finally, carrying out selective enrichment on peptide fragment ions which are pre-judged to be ultralow in abundance for multiple times, and then identifying. Therefore, the identification number of peptide fragment impurities in the high-purity polypeptide is remarkably increased.
Owner:北京行健谱实科技有限公司
Fast evaluation method and apparatus for seed cotton impurities in purchasing link
PendingCN110716033ASolve the deficiencies in existing technologies such as complicated detection procedures and slow speedImprove detection efficiencyWeighing by removing componentImage analysisAgricultural scienceAgricultural engineering
The present invention relates to an automatic detection method and apparatus, and particularly is a fast evaluation method and apparatus applied to seed cotton impurities in a seed cotton purchasing link. In an existing seed cotton purchasing process, a seed cotton impurity analytical engine is mainly used for performing an impurity separating test on seed cotton to detect a percentage of impurities, in which an impurity detection procedure is complicated and is slow in speed. As a result, a phenomenon of seed cotton transport vehicle queuing is serious. The fast evaluation method and apparatus proposed in the present invention are used for resolving a problem that a speed of detecting a percentage of impurities in seed cotton is slow in a purchasing process. The fast evaluation apparatusin the present invention comprises a pneumatic cotton delivery apparatus, a seed cotton large-impurity cleaning apparatus, a pushing apparatus, a seal capping apparatus, a conveying apparatus and an online imaging detection apparatus. The evaluation method and apparatus resolve technical problems that a procedure of detecting a percentage of impurities in seed cotton in a purchasing process is complicated and slow in speed and the like, and provides a real-time basis for seed cotton transaction settlement.
Owner:SHIHEZI UNIVERSITY
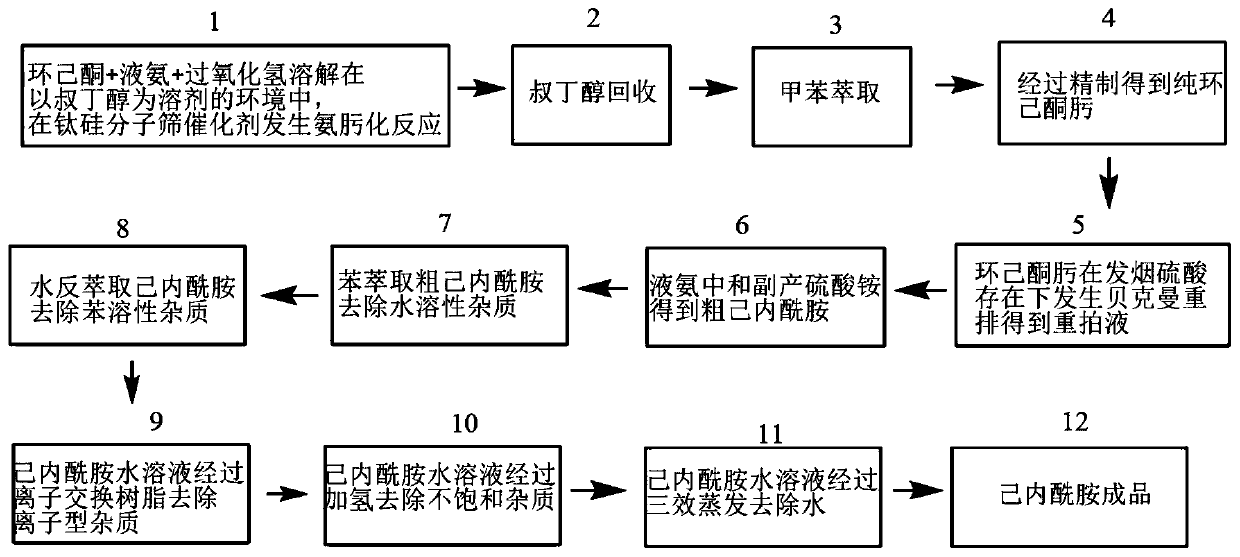
Gas chromatography detection method in caprolactam production process
ActiveCN111521723APrecise control of process parametersEasy to separateComponent separationBulk chemical productionGas liquid chromatographicPolyethylene glycol
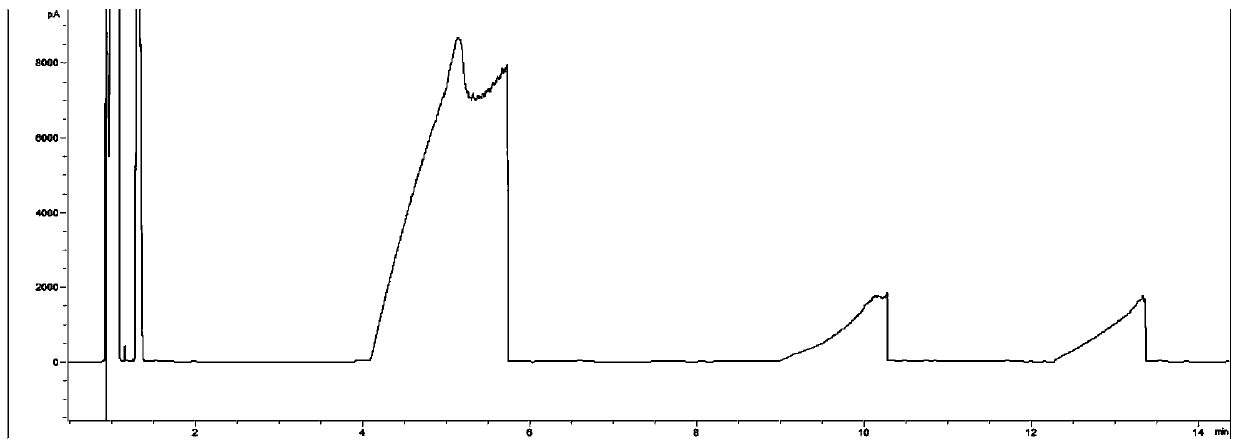
The invention relates to a gas chromatography detection method in a caprolactam production process. The method uses gas chromatography to detect an intermediate product at a quality control point in caprolactam production, and the gas chromatography uses a strongly polar chromatographic column stationary liquid selected from at least one of bonded polyethylene glycol and cross-linked polyethyleneglycol. According to the gas chromatography detection method in the caprolactam production process, the separation effect is high, and the impurity detection capacity is good. The impurity analysis capability of each control point in the caprolactam production process is improved, and caprolactam production enterprises can be better guided to carry out lean production control.
Owner:沧州旭阳化工有限公司
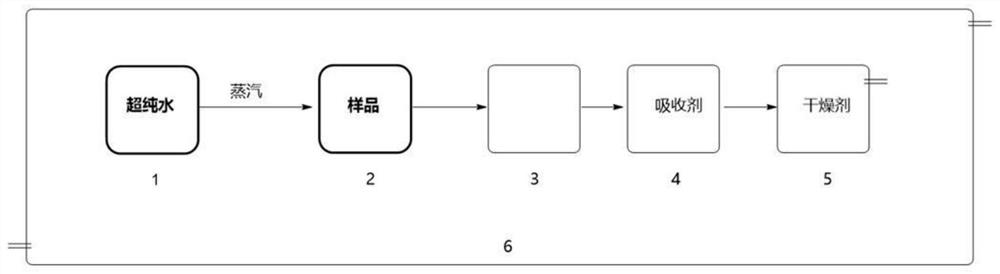
Decomposition method for analyzing trace impurities of metal organic compounds
PendingCN113075002AGood repeatabilityNo pollutionPreparing sample for investigationMaterial analysis by electric/magnetic meansWater vaporOrganic compound
The invention discloses a decomposition method for analyzing trace impurities of a metal organic compound, which comprises the following steps of feeding a dry sampling bottle and a sampling device which are accurately weighed into an inert atmosphere operation box, taking the metal organic compound by using the sampling device, and putting the metal organic compound into the sampling bottle, accurately weighing a sampling bottle and the total weight of the metal organic compounds in the sampling bottle, then sequentially connecting the sampling bottle, a buffer bottle, an absorption bottle and a drying bottle in the inert atmosphere operation box, slowly introducing ultrapure water into the sampling bottle in the form of water vapor, and after complete decomposition, preparing a corresponding to-be-detected solution by absorbing a nitric acid or hydrochloric acid solution with the mass percentage of 5% for substance in the bottle and detecting. The water vapor is adopted to decompose the metal organic source to form the soluble hydroxide, and the method has the advantages of easiness in operation, good safety, short detection time, no environmental pollution, good analysis result repeatability, wide applicability and the like.
Owner:JIANGXI NORMAL UNIV
High performance liquid chromatography analysis method for dihydralazine sulfate related substances
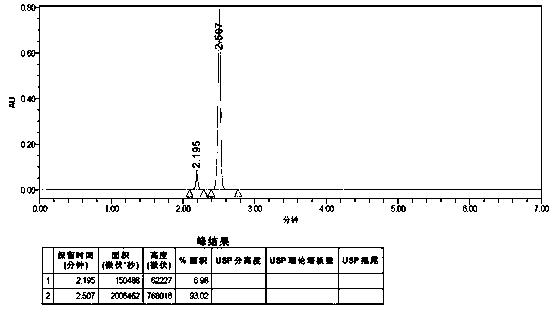
The invention discloses an ultra-high performance liquid chromatography analysis method for dihydralazine sulfate and related substances thereof. Waters UPLC AcQuity H-Class is used, a reversed-phaseC18 column is adopted, a monopotassium phosphate-phosphoric acid buffer solution is used as a mobile phase A, and methanol is used as a mobile phase B, and then gradient elute treatment is performed.According to the method, the known impurity content can be effectively controlled through a main component self-contrast method with correction factors, and the separation degree between a main peak and adjacent impurity peaks and the separation degree between the main peak and each impurity peak are both larger than 1.5. The method is simple, convenient to operate, good in reproducibility and accurate in impurity analysis and positioning, and a reliable analysis method can be provided for controlling the quality of dihydralazine sulfate and tablets thereof.
Owner:CHANGZHOU PHARMA FACTORY
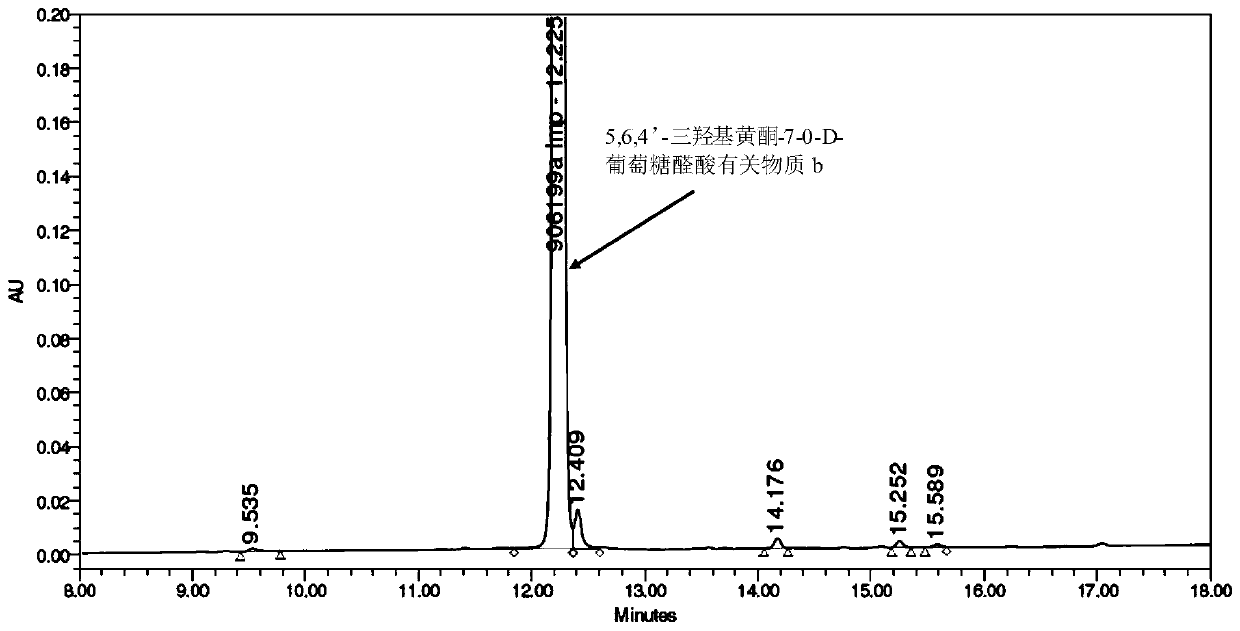
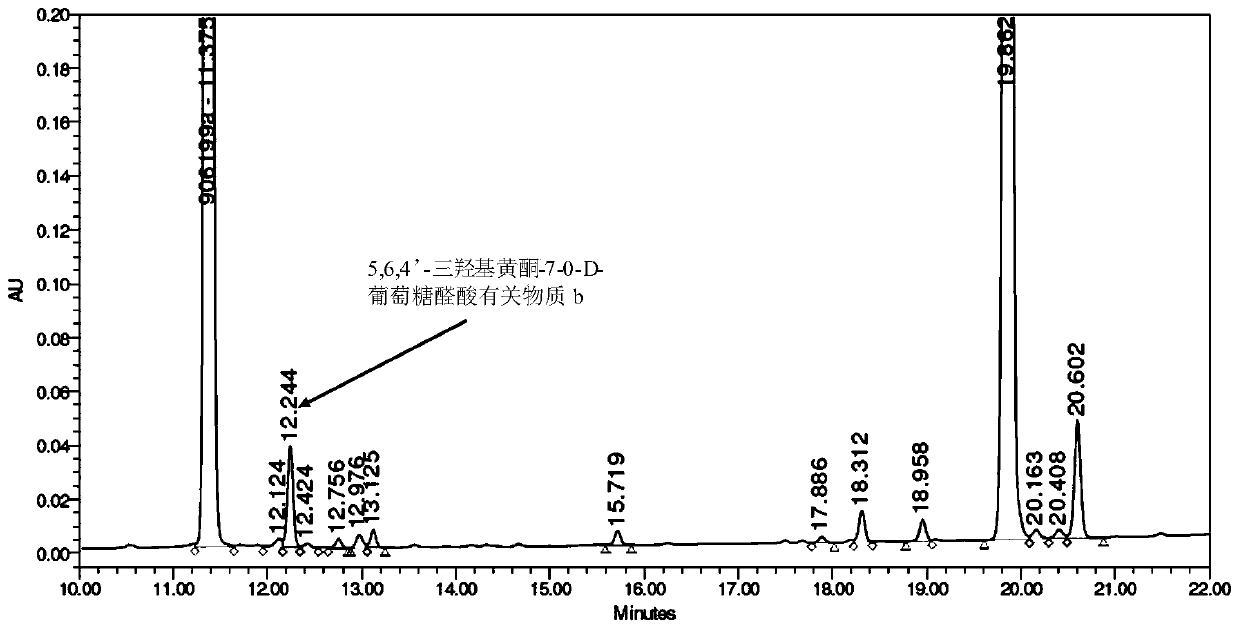
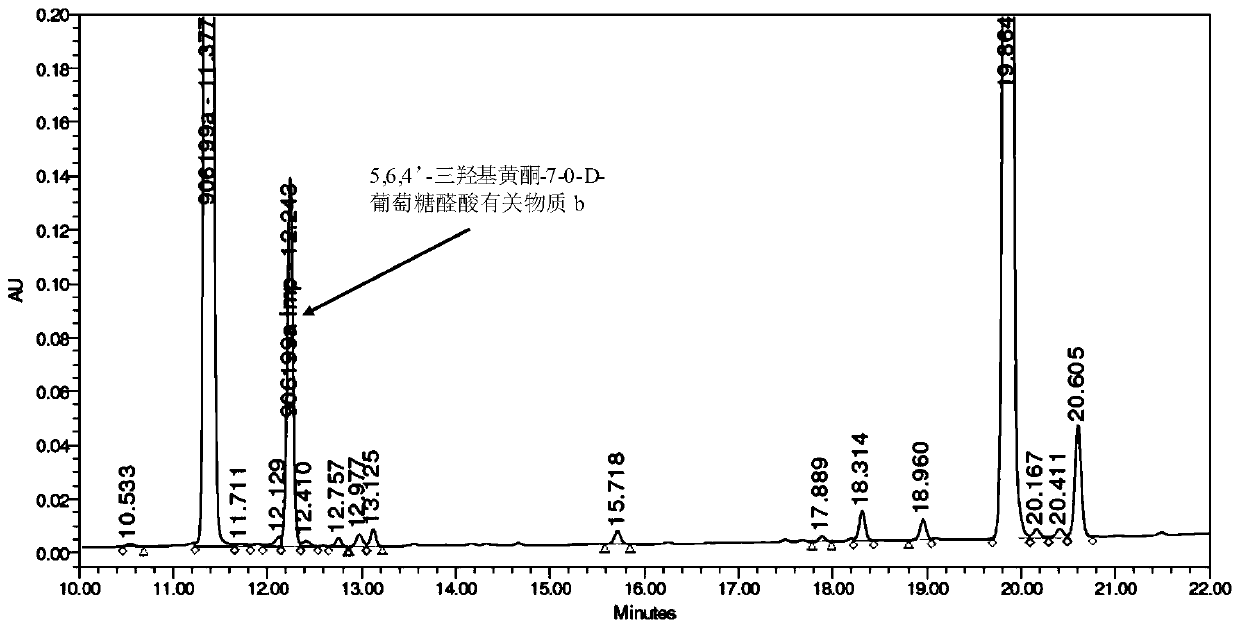
Synthesis of related substances of 5,6,4'-trihydroxyflavone-7-0-d-glucuronic acid and its preparation method and application
ActiveCN106946959BShort synthetic routeLow costSugar derivativesComponent separationUronic acidStructural formula
The invention relates to a 5,6,4'-trihydroxyl flavone-7-O-D-glucuronic acid related substance b which is successfully prepared through selective elimination and hydrolysis by taking 5,6,4'-triacetoxy flavone-7-O-D-triacetyl methyl glucuronate (the structural formula is as shown in a formula II) as a raw material. The related substance b is further purified, and finally, a 5,6,4'-trihydroxyl flavone-7-O-D-glucuronic acid related substance b monomer is obtained to obtain a related substance chemical structure after confirmation. The method is short in synthetic route, low in cost, simple to operate, accurate in result, high in product purity, high in reaction yield and easy for product purification, and can be used for impurity analysis and control of a synthesized 5,6,4'-trihydroxyl flavone-7-O-D-glucuronic acid sample, so that quality control of the synthesized 5,6,4'-trihydroxyl flavone-7-O-D-glucuronic acid is facilitated, and the product quality is better guaranteed.
Owner:KPC PHARM INC
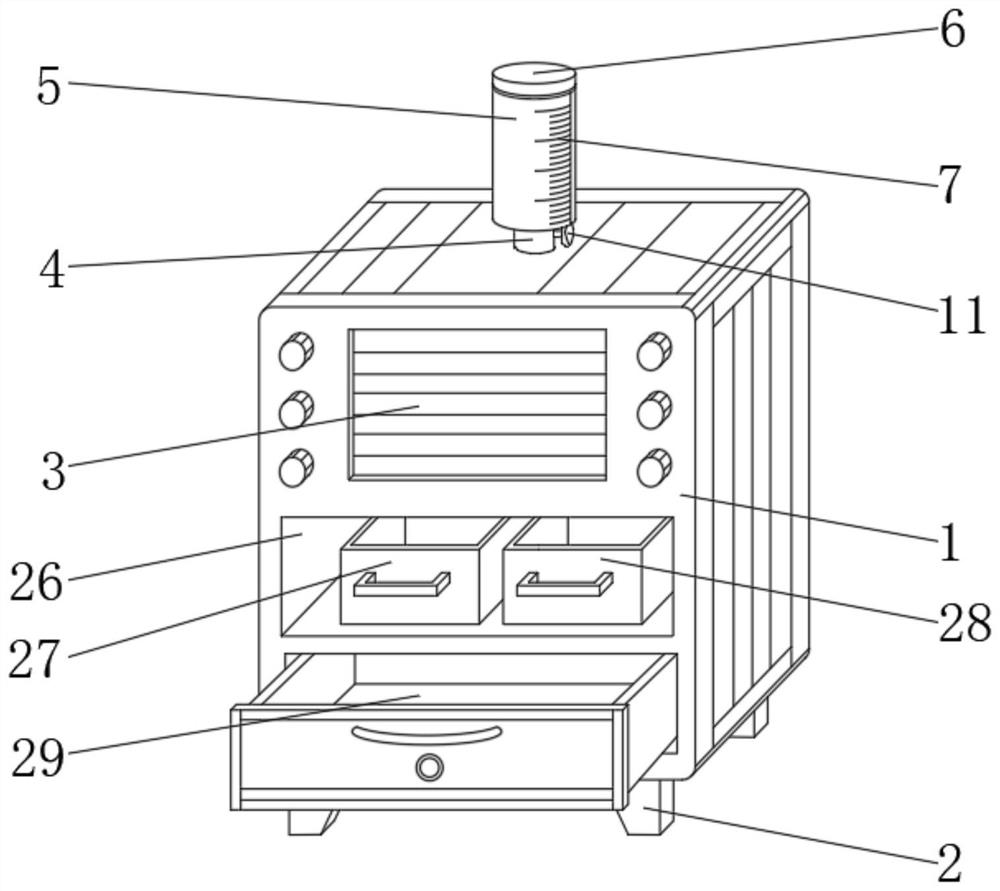
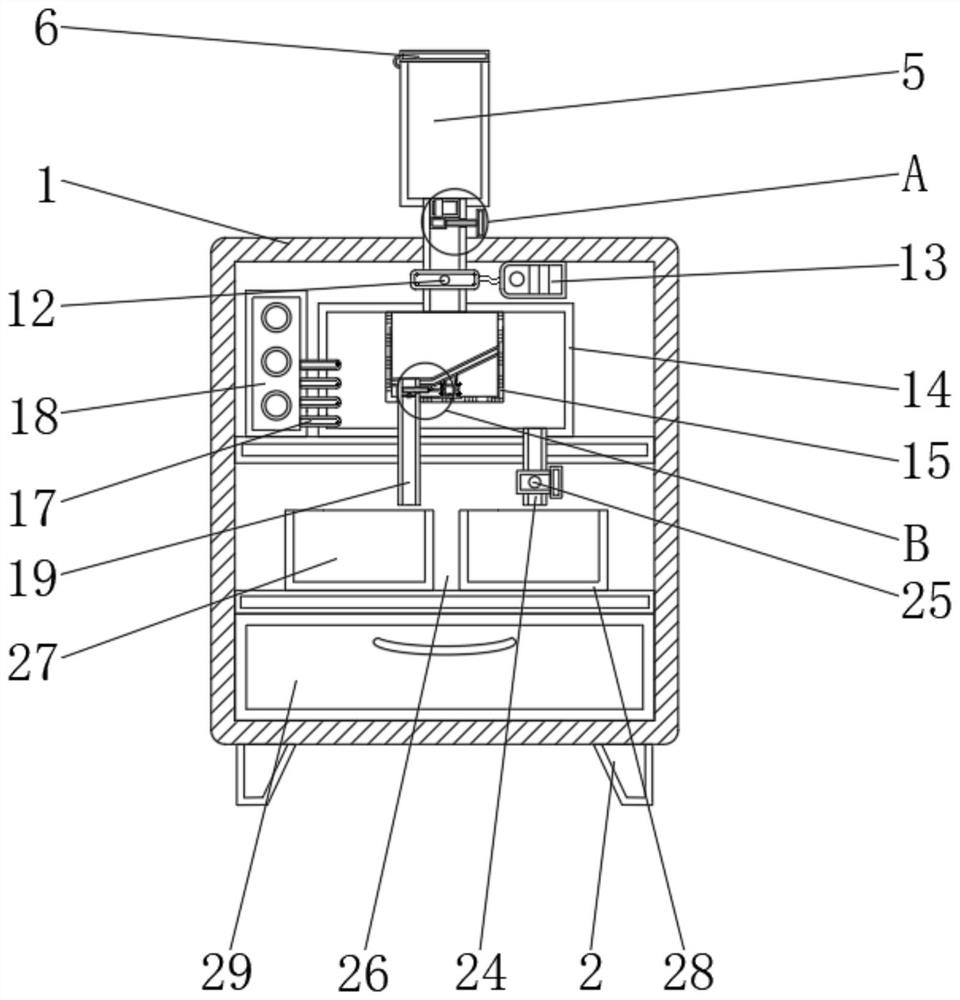
Impurity analysis instrument for chemical detection
ActiveCN113138112AEasy to observeFunction as a quantitativePreparing sample for investigationPhysical chemistryEngineering
The invention discloses an impurity analysis instrument for chemical detection, and relates to the technical field of chemical detection, in particular to an impurity analysis instrument for chemical detection, which comprises an analyzer shell, wherein support legs are fixed below the analyzer shell; a display screen embedded in the outer wall of the analyzer shell; a feeding pipe arranged above the analyzer shell, wherein the upper part of the feeding pipe is connected with a quantitative box, and the feeding pipe is communicated with the quantitative box; a metal detection head fixedly arranged on the outer wall of the feeding pipe; and an analysis box. According to the impurity analysis instrument for chemical detection, the quantitative box is arranged, industrial impurities are put into the quantitative box by opening a protective cover, the industrial impurities in the quantitative box can be conveniently observed under the action of a dial gauge, and the protective cover is closed after the industrial impurities are put to a proper amount, so that the quantitative function is achieved; and the situation that needed detection data cannot be accurately obtained due to the fact that too many industrial impurities are put is avoided, and practicability is high.
Owner:贵州省产品质量检验检测院
Method for cleaning gas chromatographic column for analyzing trace impurities in high-purity carbonic ester
InactiveCN112792034AWill not harmIncrease contentComponent separationCleaning using liquidsOrganic solventGas liquid chromatographic
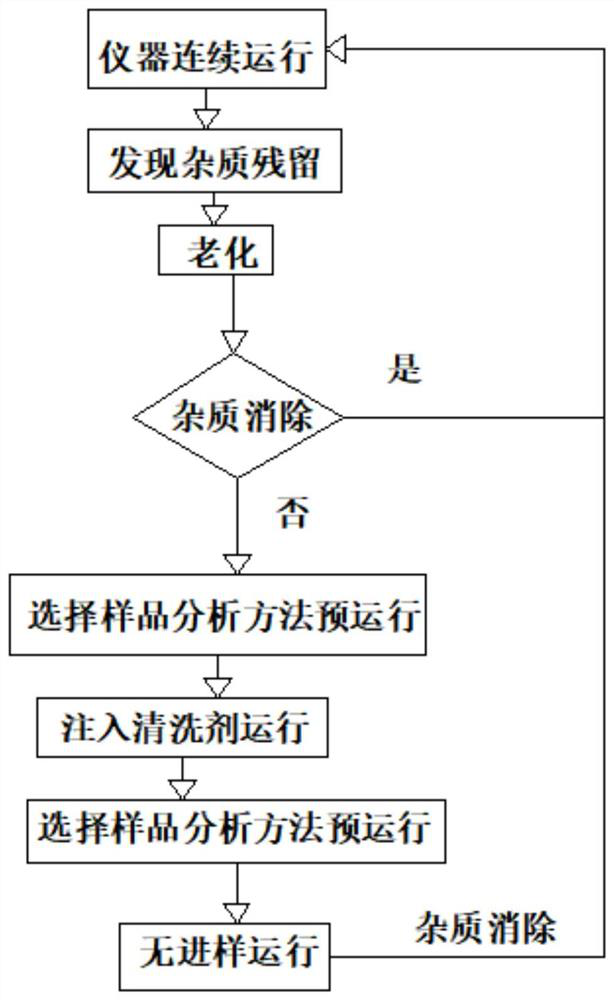
The invention discloses a method for cleaning a gas chromatographic column for analyzing trace impurities in high-purity carbonic ester. The method aims to solve the problem that measurement accuracy is influenced by chromatographic column residues in analysis of trace impurities in high-purity carbonic ester, a sample injection cleaning method is adopted, the method is a chromatographic method for keeping sample analysis, and a cleaning agent is injected into a chromatograph for operation, and then one-time non-injection operation is executed. The cleaning agent is an organic solvent capable of dissolving the carbonic ester to be detected, is extremely high in content and lower in boiling point, and does not easily cause component residues; and meanwhile, trace impurity residues can be dissolved out, and the trace impurity residues can be completely eliminated through sample injection-free operation. The method does not need to disassemble and assemble an instrument, is simple to operate and short in time consumption, does not damage the chromatographic column, and has a cleaning elimination rate of trace impurity residues greater than 99%.
Owner:江苏思派新能源科技有限公司
Method for determining impurity content of recycled plastic and application of method
PendingCN114705516AThe difference in impurity content will not be too largeReduce measurement biasComponent separationMaterial heat developmentPhysical chemistryQualitative analysis
Owner:上海睿聚环保科技有限公司
Method for detecting purity of pimpinolide
InactiveCN110954643AHigh sensitivityEasy to separateComponent separationColumn temperatureGradient elution
The invention discloses a method for detecting the purity of pimpinolide. The invention belongs to the technical field of traditional Chinese medicine purity detection. The method comprises the following steps: (1) preparation of a test solution: precisely weighing 1.75-3.0 mg of a pimpinolide test sample, putting the test sample into a 1mL or 3mL volumetric flask, fixing the volume to a scale byusing a 50% acetonitrile-water mixed solution, uniformly shaking, passing through a 0.22 mu m microfiltration membrane or centrifuging, and taking the supernatant for later use; (2) detecting the testsolution by using ultra-high performance liquid chromatography and gradient elution, wherein the detection conditions are as follows: a chromatographic column is a reversed-phase C18 column; the column temperature of the chromatographic column is 20-45 DEG C; a mobile phase: the mobile phase A is methanol or acetonitrile; the mobile phase B is water; wherein the flow rate is 0.2 mL / min to 0.5 mL / min; the detector is a diode array detector, and the detection wavelength of the diode array detector is 254-330nm; wherein the sample size of the pimpinolide test solution is 0.2 mu L-1. 0mu L. The invention further discloses a detection method of the pimpinolide. The method provided by the invention has the characteristics of accuracy, sensitivity and quickness, provides reliable guarantee for impurity analysis of the pimpinolide, and also saves time and material cost.
Owner:成都普思检验检测有限公司
Method for Impurity Analysis of Multivitamin Preparations
ActiveCN109239230BLong retention timeStrong UV Absorbing PropertiesComponent separationBenzoic acidBoronic acid
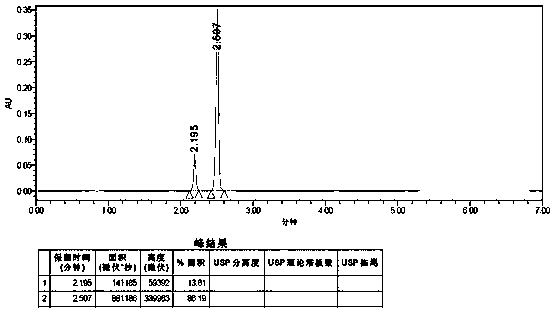
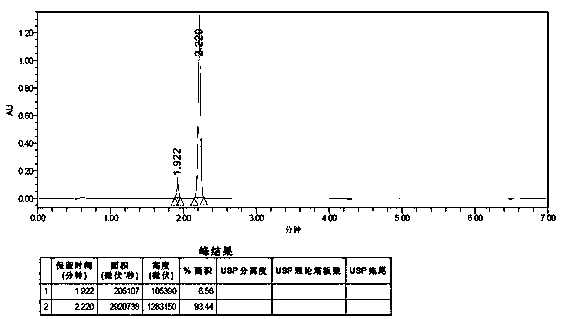
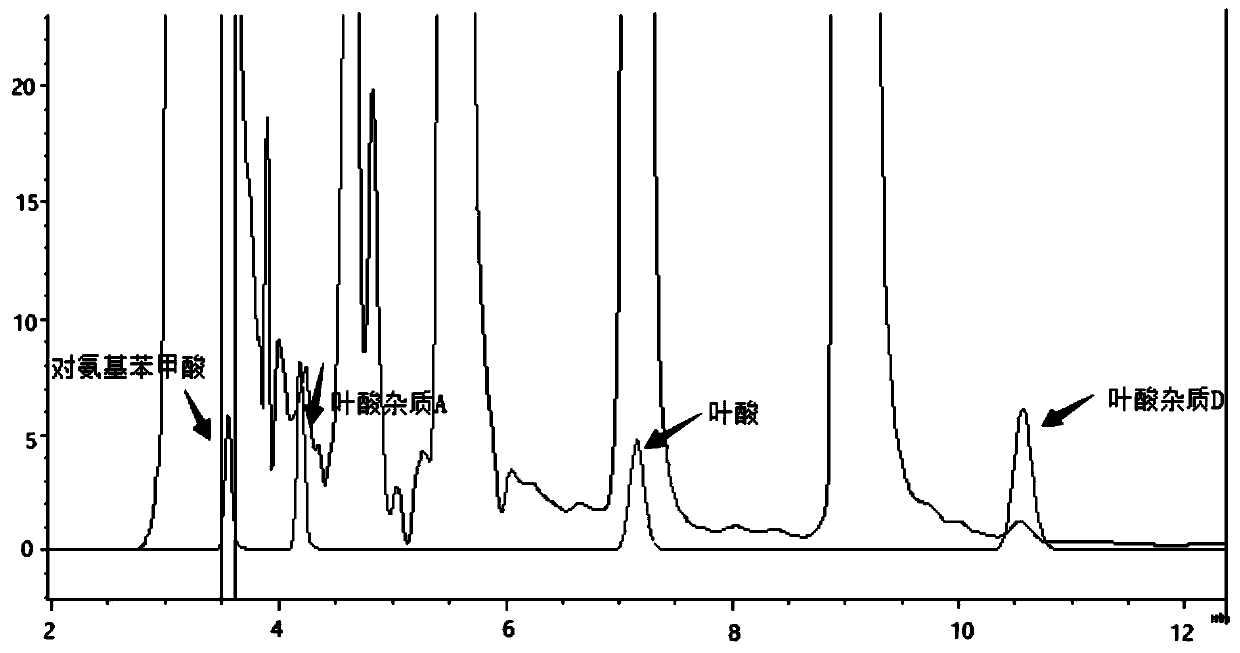
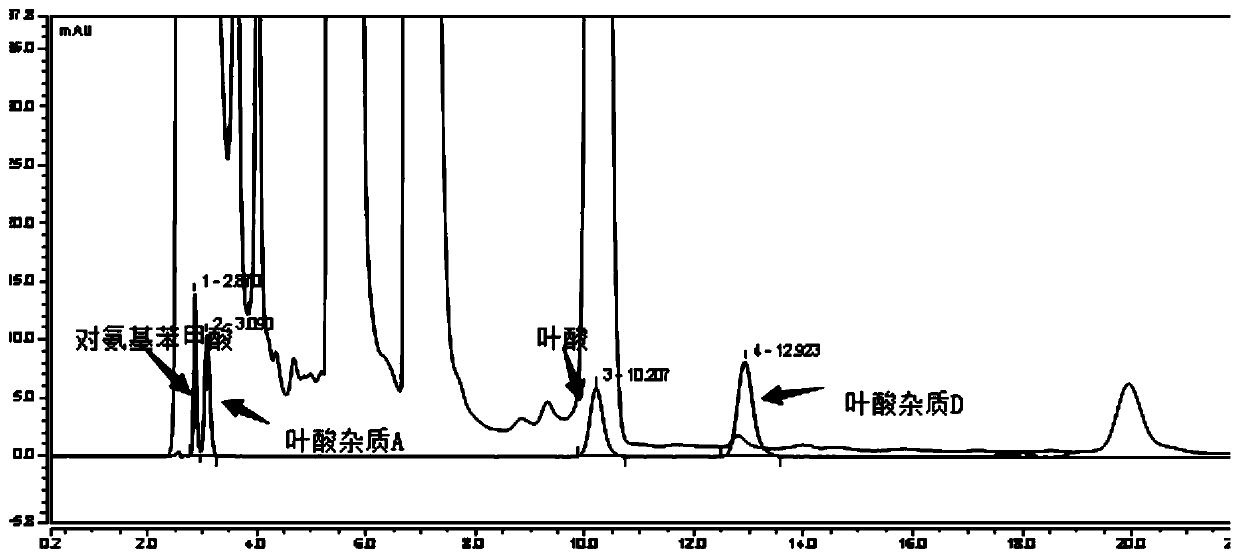
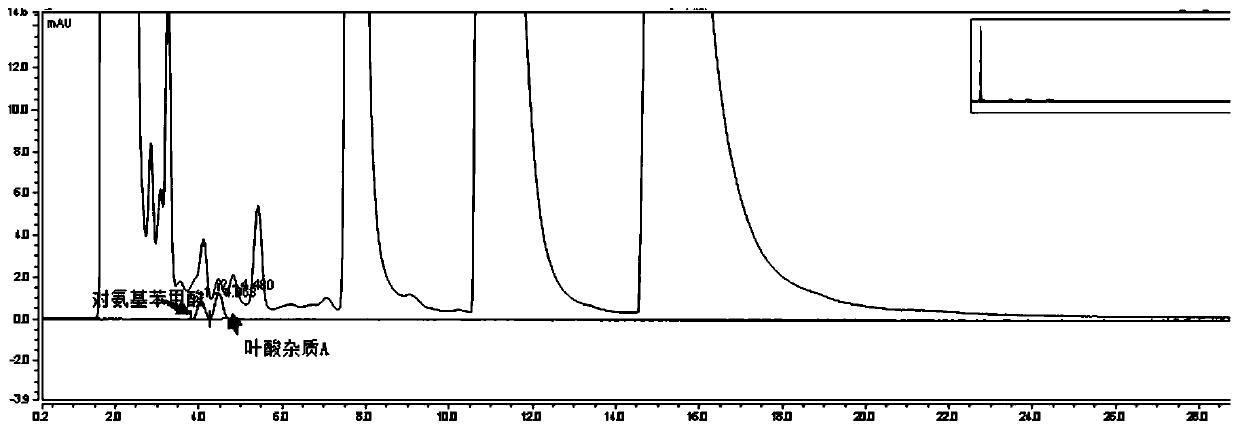
The invention relates to an impurity analysis method for multiple vitamin preparations. The method comprises the following steps of preparing a test solution of the vitamin preparations; dissolving o-phthalaldehyde and 2-mercaptoethanol based on a mass ratio of 1 to (2-5) in methanol, adding a boric acid buffer solution, and adjusting the pH value to 3-5 to obtain a derivatization reagent; and taking the test solution, adding the derivatization reagent to carry out online derivatization reaction, and injecting the derivatized test solution into a high performance liquid chromatograph, therebydetecting impurities in the test solution. According to the method, folic acid impurity A, folic acid impurity D, p-aminobenzoic acid and 3-aminopropanol in the vitamin preparations can be effectivelydetected; and the method is high in sensitivity and good in specificity.
Owner:CHINESE MEDICINES GUANGZHOU
A method for improving the detection sensitivity of special impurities in high-purity hydrogen chloride
ActiveCN109507321BHigh detection sensitivityEasy to separateComponent separationPhysical chemistryHelium ions
The invention relates to the field of high-purity gas impurity detection and specifically relates to a method for increasing detection sensitivity of special impurities in high-purity hydrogen chloride. According to the method for increasing detection sensitivity of special impurities in high-purity hydrogen chloride disclosed by the invention, two parallel chromatographic columns are used; an independent temperature-control column box is adopted, so that column temperatures of the two parallel chromatographic columns can be guaranteed, and such an arrangement is beneficial to respectively separated components; a helium ion detector is used for detecting trace impurities and the limit of detection can be reduced to 1-10ppb; a filler special for high-purity hydrogen chloride chromatographicanalysis and nanometer titanium dioxide are adopted for modifying a capillary column, so that the separation of trace impurities in electronic hydrogen chloride can be boosted; the helium ion detector is used for detecting hydrogen chloride according to the method, so that the sensitivity is high and the limit of detection is low; impurity component response is linear; after a gas flow is designed, sample can be injected at one time and the requirements for analyzing all the impurities of electronic hydrogen chloride gas can be met.
Owner:ZHEJIANG BRITECH CO LTD
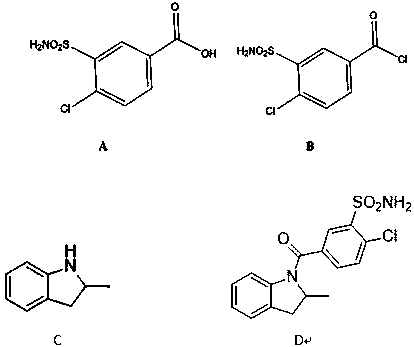
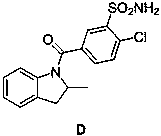
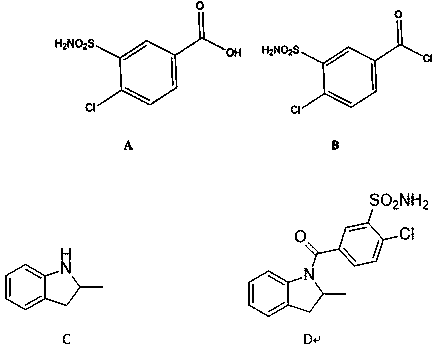
Impurity generated in production of indapamide as well as synthesis method and application
PendingCN111170922ASolve analytical control problemsOrganic chemistryComponent separationDistillationPhysical chemistry
The invention discloses an impurity generated in production of indapamide as well as a synthesis method and application. The synthesis method of the indapamide impurity D comprises the following stepsof: reacting an initial raw material A with thionyl chloride, and carrying out reduced pressure distillation to obtain a compound B, carrying out acylation reaction on the compound B and C, and recrystallizing petroleum ether and ethyl acetate to obtain a compound D. The invention provides a simple synthesis method of the indapamide impurity D. The synthesized indapamide impurity D can provide impurity contrast monitoring for reaction, and the problem of impurity analysis and control of indapamide is solved. And a strong support is provided for large-scale industrial production of indapamide.
Owner:TIANJIN LISHENG PHARM CO LTD
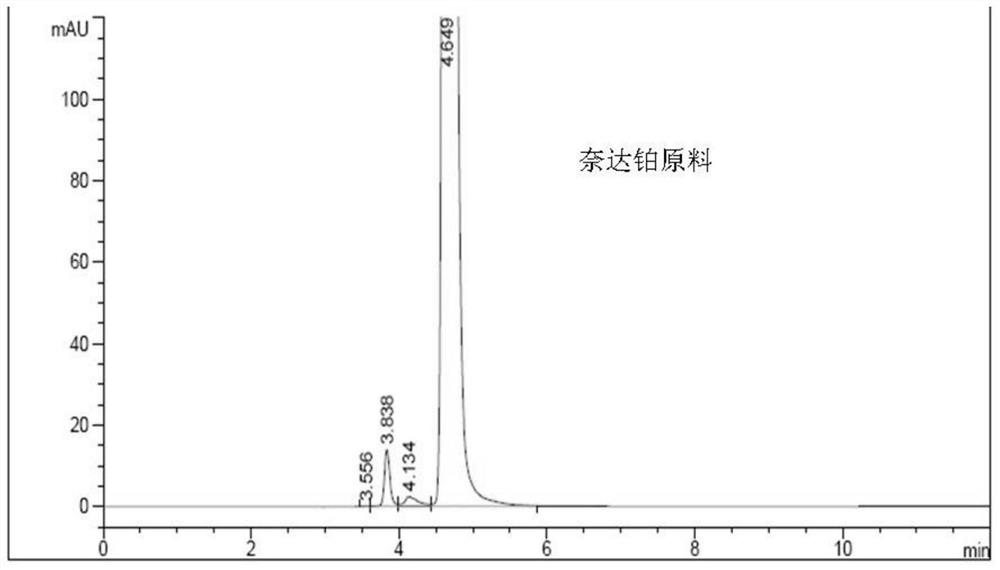
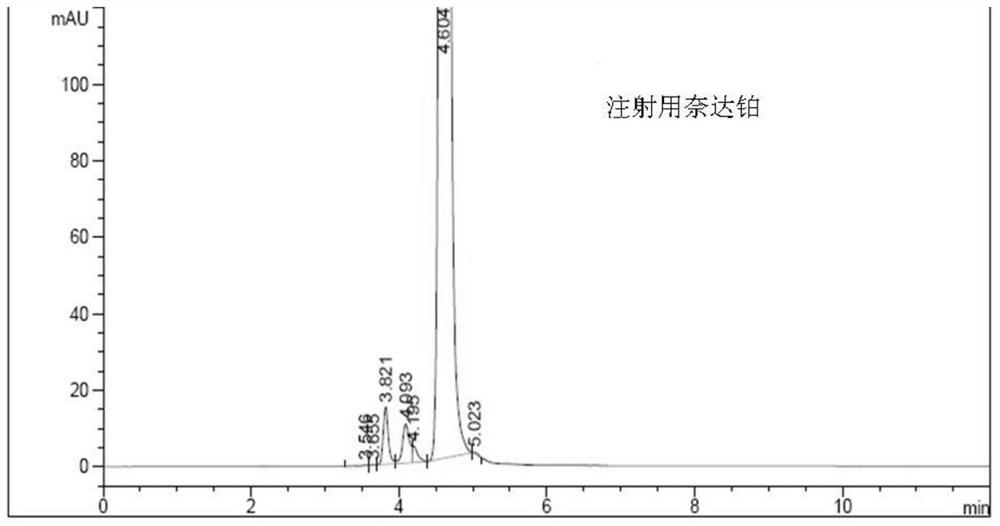
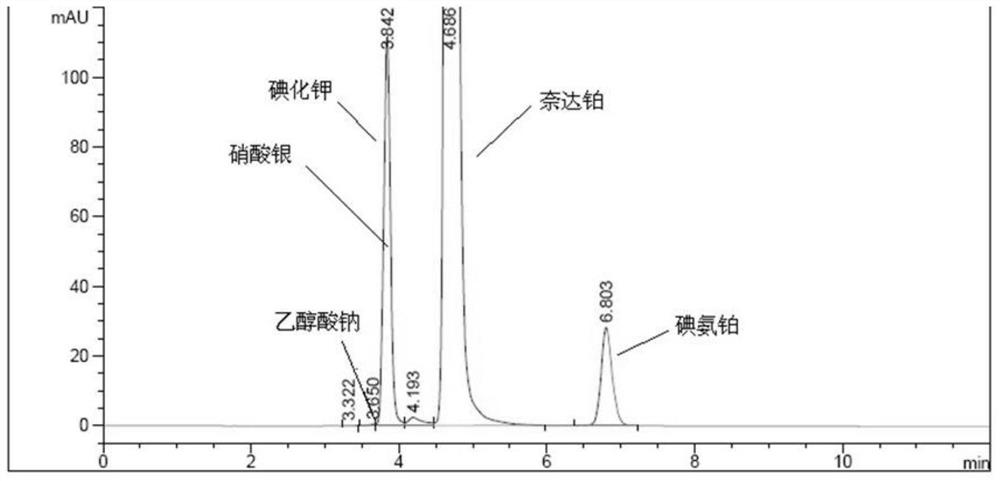
A kind of impurity detection method of nedaplatin
The invention discloses an impurity HPLC analysis method for nedaplatin. In the method, octadecylsilane bonded silica gel is used as a filler (recommendation: AQ-C18), the detection wavelength is 205-222nm, and the column temperature is 20~45℃, the mobile phase is a mixture of pH6.0~8.5 buffer and polar organic solvent, characterized in that the mobile phase is to elute the impurities in the nedaplatin sample by gradient elution, pH6. The initial ratio of 0-8.5 buffer solution to polar organic solvent is 100:0-90:10, and the final ratio is 90:10-60:40; the flow rate of mobile phase is 0.5-1.0ml / min; prepared with mobile phase into a solution containing 0.5 mg to 2 mg / ml of nedaplatin; inject 5 μl to 20 μl of the sample solution into a high performance liquid chromatograph, record the chromatogram and perform sample analysis. After using the HPLC analysis method of the present invention, the theoretical plate number of the main peak of the product is obviously increased, and more impurities can be separated, so that the accuracy of analyzing the nedaplatin sample is improved.
Owner:SIMCERE ZAIMING PHARM CO LTD
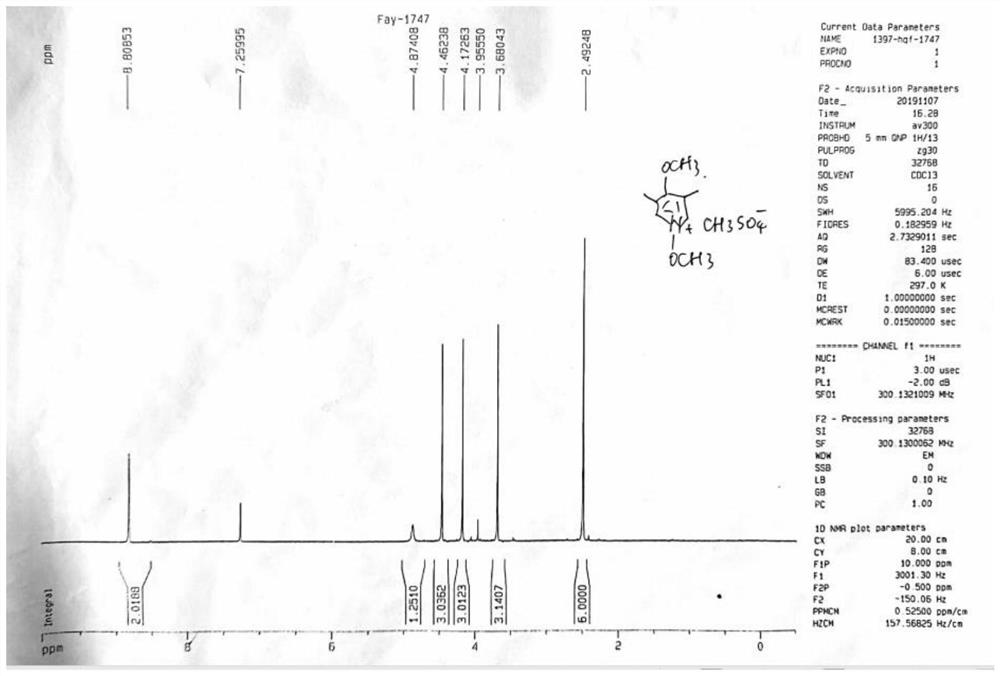
Omeprazole process impurity and preparation method thereof
The present invention relates to an omeprazole process impurity and a preparation method thereof, the omeprazole process impurity has a structure represented by a formula I: wherein X<-> is CH3SO4<->or HSO4<->. Separation, confirmation and preparation of the omeprazole process impurity are beneficial to impurity analysis of an omeprazole intermediate and an omeprazole bulk drug, and improvement of a synthesis process of the omeprazole intermediate and the omeprazole bulk drug, so that further improvement of the quality standard and control of the omeprazole bulk drug is facilitated; the quality level of the raw material medicine is improved, and guarantee is provided for the medication safety of people.
Owner:成都百泉生物医药科技有限公司
Device and method for analyzing impurities in hydrogen isotope gas
ActiveCN114544807ARemove overlapImprove analysis accuracyComponent separationNuclear energy generationHydrogen purifierNitrogen gas
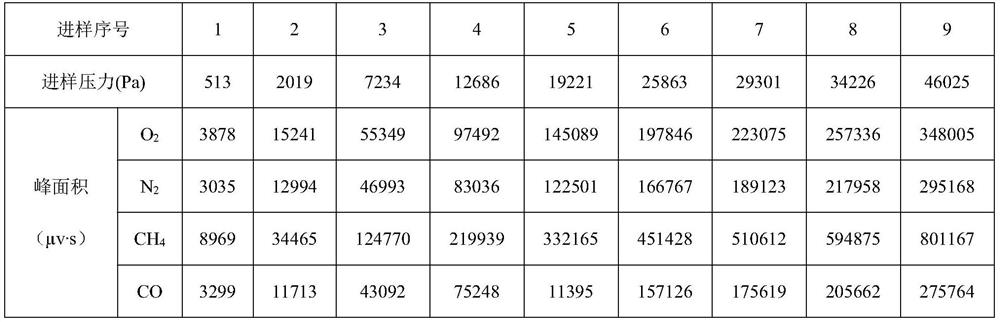
The invention discloses a device for analyzing impurities in hydrogen isotope gas, which comprises a carrier gas source, a standard gas source, a sample container, a chromatographic column box, a hydrogen purifier and a thermal conductivity detector, the chromatographic column box comprises a first chromatographic column and a second chromatographic column, and the hydrogen purifier can adsorb the hydrogen isotope gas. According to the invention, the hydrogen purifier is connected in series in front of the first chromatographic column, and the hydrogen purifier can adsorb hydrogen isotope gas below the detection limit of the thermal conductivity detector so as to eliminate the overlap between deuterium and tritium peaks and helium peaks, so that the analysis precision of helium impurities in the hydrogen isotope gas is improved; the invention further provides a method for analyzing the impurities in the hydrogen isotope gas, argon is used as carrier gas, analysis is carried out through the second chromatographic column, and therefore interference of argon impurities in a hydrogen isotope sample on analysis of oxygen impurities is avoided. According to the invention, high-precision analysis of impurities such as nitrogen, methane and carbon monoxide is realized while high-precision analysis of impurities such as helium and oxygen in the hydrogen isotope gas is realized.
Owner:MATERIAL INST OF CHINA ACADEMY OF ENG PHYSICS
Sitagliptin key intermediate impurity as well as preparation method and application thereof
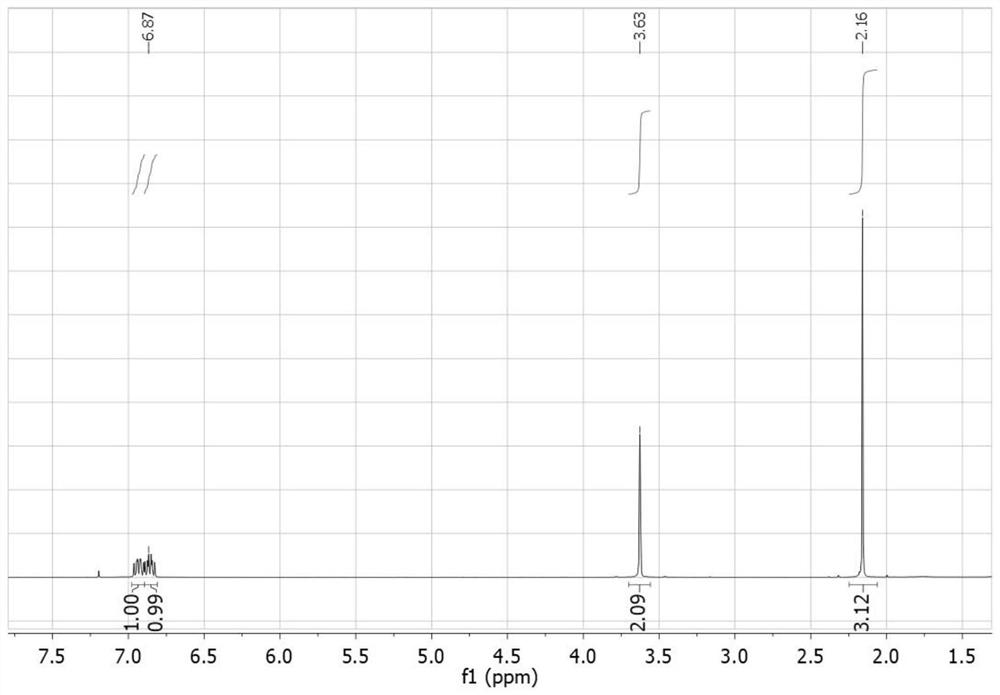
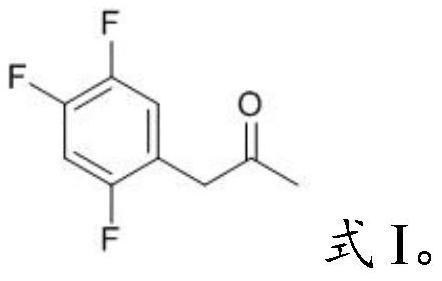
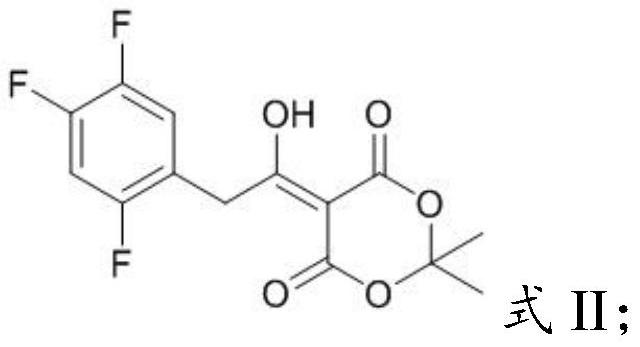
PendingCN112194576AEasy to operateSimple methodPreparation from heterocyclic compoundsPharmacologyImpurity profiling
The invention provides a sitagliptin key intermediate impurity as well as a preparation method and application thereof, which belong to the technical field of medicinal chemistry. The compound with the structure as shown in the formula I provided by the invention is an impurity component generated in the production process of a key intermediate acetyl Meldrum's acid derivative 5-(1-hydroxy 2-(2, 4, 5-trifluorophenyl)ethylene)-2, 2-dimethyl-1, 3-dioxepin-4, 6-diketone) (II) for synthesizing sitagliptin. The impurity can be used as a reference substance to be applied to impurity analysis of keyintermediates in a sitagliptin production process so as to promote quality control of the key intermediates and further effectively control the quality of sitagliptin drugs. The invention provides thepreparation method of the sitagliptin key intermediate impurity, the synthesis method of the impurity reference substance of the sitagliptin key intermediate is established through pyrolysis, the method is a basis for researching impurity composition and quality control of sitagliptin, and the method is strong in operability and simple.
Owner:TAIZHOU BIOMEDICAL & CHEM IND RES INST CO LTD
Method for separating and analyzing impurities in NF3
ActiveCN112485348AEffective protectionShort analysis timeComponent separationNuclear energy generationGas liquid chromatographicDischarge ionization detector
The invention discloses a method for separating and analyzing impurities in NF3, which adopts gas chromatography with double blowback or double cutting systems, takes high-purity helium as carrier gas, configures a helium discharge ionization detector, and comprises the step of emptying main components by using a blowback switching valve after the impurities flow out of a pre-column. According tothe method for separating and analyzing the impurities in the NF3, the impurities are separated, the analysis time is short, and the separation effect is remarkable. A large amount of NF3 can be prevented from entering the detector, and the detector is effectively protected.
Owner:北京高麦克仪器科技有限公司
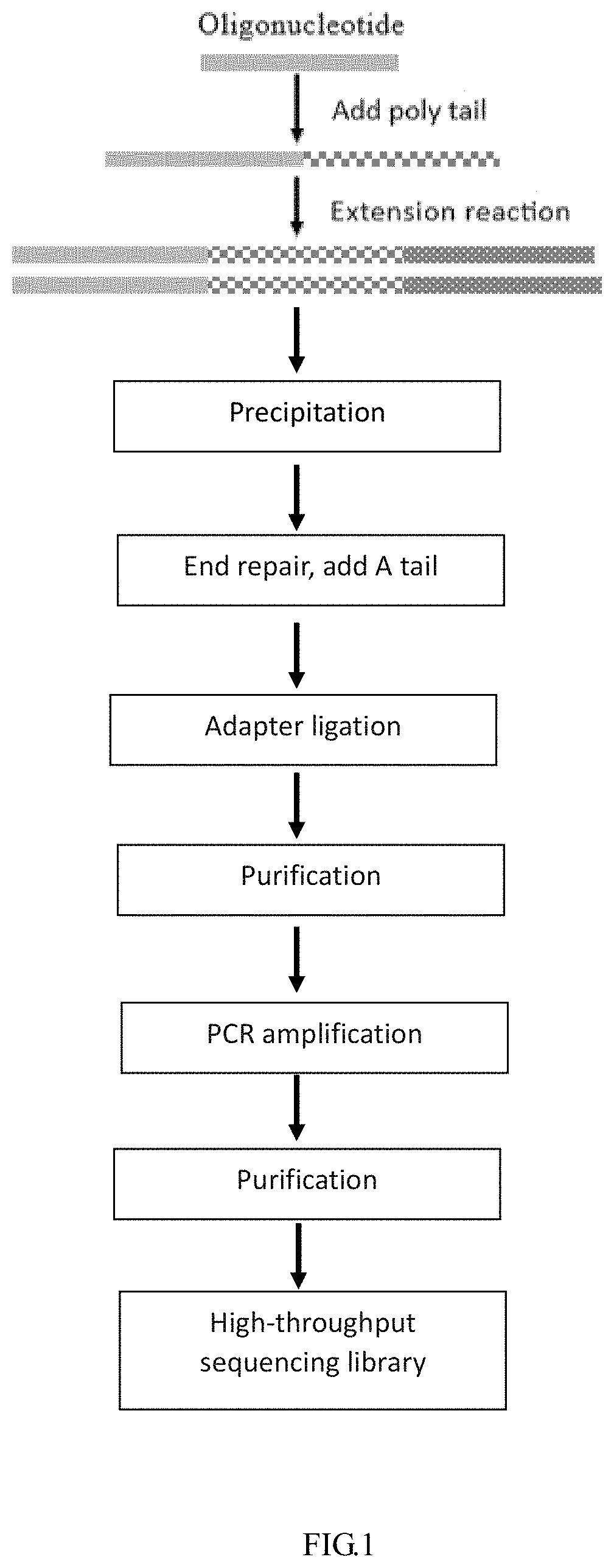
Method for analyzing impurities of oligonucleotide sequence based on high-throughput sequencing and application
The present invention provides a method for analyzing impurities of an oligonucleotide sequence based on high-throughput sequencing. The method of the present invention comprises the following steps: constructing a high-throughput sequencing library for analysis of impurities of an oligonucleotide sequence; subjecting the high-throughput sequencing library to high-throughput sequencing, and analyzing the nucleotide sequence components according to the sequencing results; the sequence of the extension primer used in the construction of the high-throughput sequencing library consisting of the DNA molecule set forth in positions 1-22 of SEQ ID NO: 2 and N bases (A, T, C or G) in sequence; and N being an integer greater than or equal to 6. It is proved by experiments that the method for analyzing impurities of an oligonucleotide sequence based on high-throughput sequencing of the present invention can quickly, accurately, and comprehensively analyze the purity and content of each component in the oligonucleotide sequence.
Owner:SUZHOU GENESCI CO LTD +1
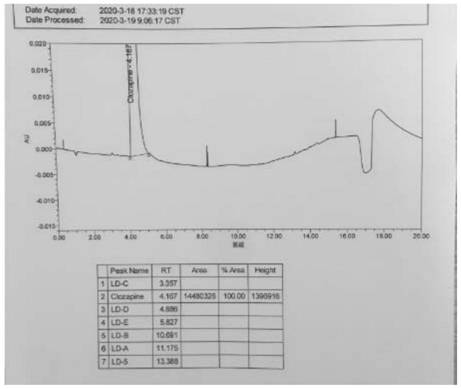
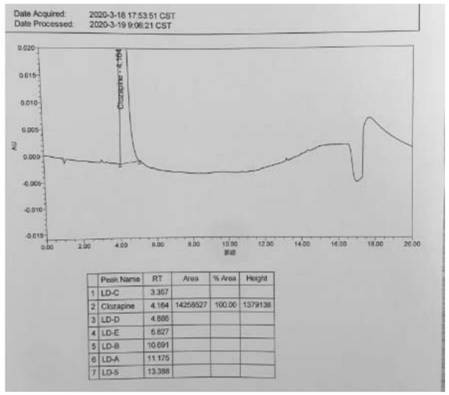
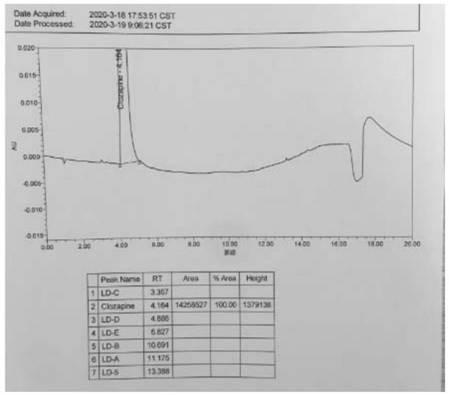
A kind of ultra-high performance liquid chromatography analysis method of clozapine related substances
The invention discloses an ultra-high performance liquid chromatography analysis method for clozapine, which uses Waters UPLC AcQuity H-Class, adopts Agilent Rapid Resolution HD column, and uses methanol-potassium dihydrogen phosphate-water as mobile phase to carry out gradient elution. This method can simultaneously analyze all known impurities in clozapine raw materials and their preparations, and can effectively control the content of known impurities through the main component self-contrast method with correction factor, the main peak and adjacent impurity peaks and the difference between each impurity peak. The degree of separation was greater than 1.5. The method is simple, time-consuming and accurate in impurity analysis, providing a reliable analytical method for clozapine.
Owner:CHANGZHOU PHARMA FACTORY
Ultra-high performance liquid chromatography analysis method of clozapine related substances
The invention discloses an ultra-high performance liquid chromatography analysis method of clozapine. The method comprises the following steps: carrying out gradient elution by using a Waters UPLC (Ultra Performance Liquid Chromatography) AcQuity H-Class, adopting an Agilent Rapid Response HD column and taking methanol-potassium dihydrogen phosphate-water as a mobile phase. According to the method, all known impurities in the clozapine raw material and the preparation thereof can be analyzed at the same time, the content of the known impurities can be effectively controlled through a main component self-control method with correction factors, and the separation degree between a main peak and adjacent impurity peaks and the separation degree between all impurity peaks are larger than 1.5. The method is simple, short in time and accurate in impurity analysis, and a reliable analysis method is provided for clozapine.
Owner:CHANGZHOU PHARMA FACTORY
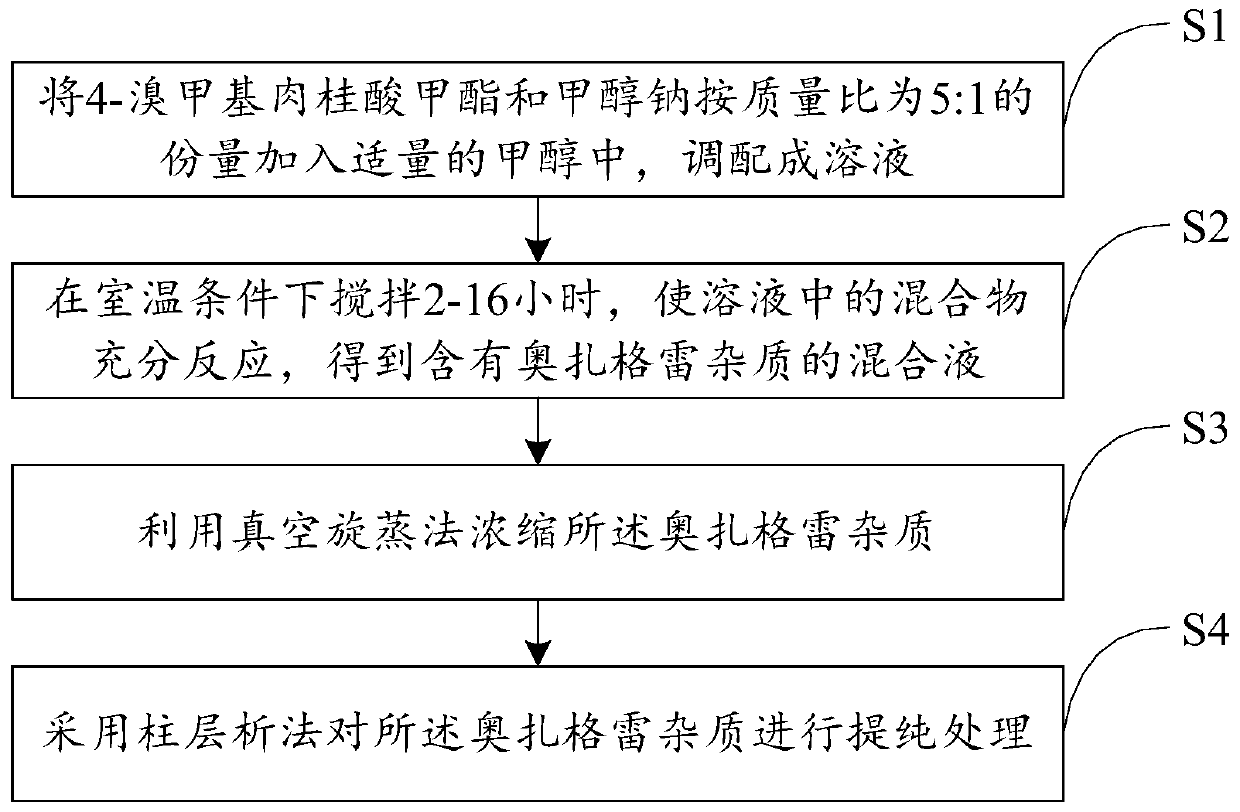
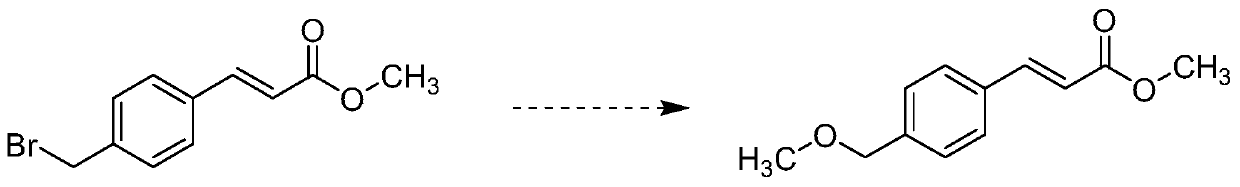
Preparation method of ozagrel impurity
InactiveCN110862319AEasy to analyzeConvenient researchOrganic compound preparationCarboxylic acid esters separation/purificationSodium methoxideMethyl palmoxirate
The invention relates to a preparation method of a zagrel impurity. The method includes the steps of: adding methyl 4-bromomethylcinnamate and sodium methoxide into a proper amount of methanol according to a mass ratio of 5:1, and blending the substances into a dilute solution; performing stirring for 2-16h under a room temperature condition to enable full reaction of the mixture, thus obtaining amixed solution containing ozagrel impurity; concentrating the ozagrel impurity by vacuum rotary evaporation method; and carrying out purification treatment on the ozagrel impurity by column chromatography process. The method provided by the invention can simply and directly acquire an ozagrel impurity reference substance, also provides convenience for impurity analysis and research of ozagrel crude drugs and preparations thereof, and provides a detection method and judgment basis for production and medication safety of ozagrel.
Owner:深圳振强生物技术有限公司
Preparation method for sarpogrelate hydrochloride photodegradable impurity III
InactiveCN105237411AHigh purityEasy to operateOrganic compound preparationAmino-hyroxy compound preparationSarpogrelate HydrochlorideEsterification reaction
The invention belongs to the field of synthesis of medicines, and specifically relates to a preparation method for a sarpogrelate hydrochloride photodegradable impurity III. The sarpogrelate hydrochloride photodegradable impurity III is prepared by comprising five-step reactions of substitution reaction, addition reaction, esterification reaction, epoxidation reaction and reduction reaction with 2-[2-(3-methoxyphenyl)vinyl]phenol as a raw material; and after column chromatography separation of a crude sarpogrelate hydrochloride photodegradable impurity III, purity reaches more than 99%. The preparation method provided by the invention has simple operation and mild reaction; the sarpogrelate hydrochloride photodegradable impurity III has high purity and can be applied in analysis of impurity profiling; and total yield is high.
Owner:SHANDONG QIDU PHARMA
A kind of high performance liquid chromatography analysis method of dihydralazine sulfate related substances
Owner:CHANGZHOU PHARMA FACTORY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com