Omeprazole process impurity and preparation method thereof
A process impurity, omeprazole technology, applied in the field of medicine, can solve the problems of difficult quality research, clinical drug safety monitoring, separation, structure confirmation and synthesis preparation, and difficult source of drug impurity reference substances, so as to achieve perfect quality Standards and controls, improving quality standards, and improving the effect of quality levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The generation, separation and identification of embodiment 1 impurity
[0040] In this example, omeprazole intermediate 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride was prepared with reference to the method in patent US4544750A.
[0041] Step A: Synthesis of Compound A:
[0042]
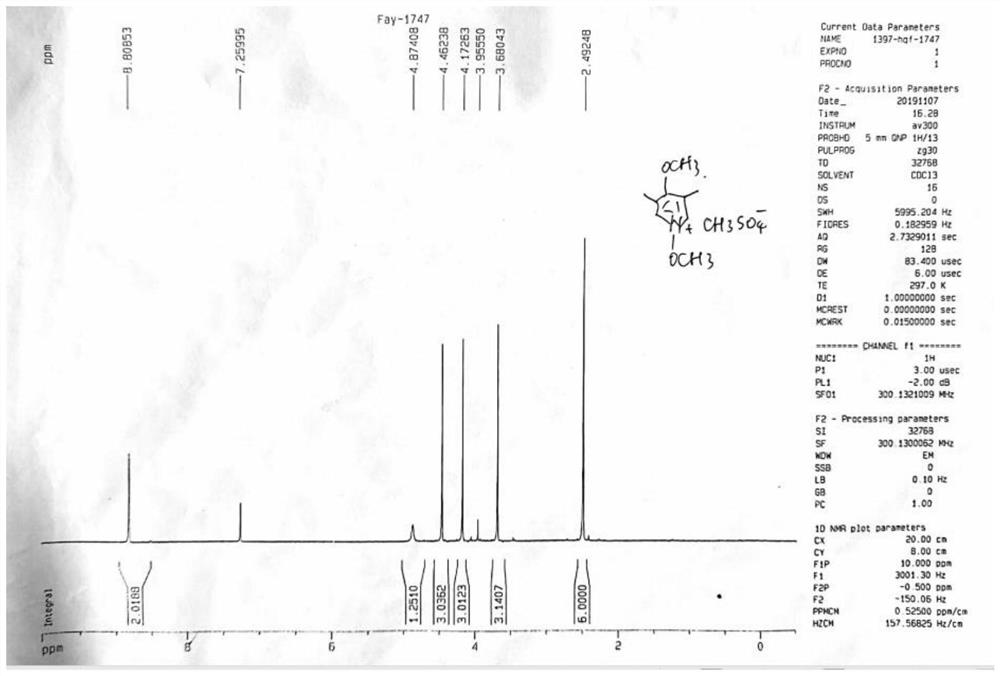
[0043] Take 3,5-dimethyl-4-methoxypyridine nitrogen oxide (35.9g, 0.235mol) in a one-necked bottle, add 145mL of chloroform and stir to dissolve, raise the temperature to 60°C, add dropwise dimethyl sulfate (24.4mL , 0.258mol), after 5 hours, the heating was turned off to stop the reaction. Cool down, add water to extract, and evaporate to dryness to obtain 65.0 g (23.29 mmol) of Compound A as a solid, with a yield of 99.1%. Common NMR spectrum such as figure 1 shown.
[0044] Step B: Synthesis of Compound B
[0045]
[0046] Take compound A (4.9g, 17.56mmol) and add 20mL of methanol, stir and dissolve evenly, and heat up to reflux. Ammonium persulfate solution (4.0...
Embodiment 2
[0068] The preparation of embodiment 2 impurity I
[0069]
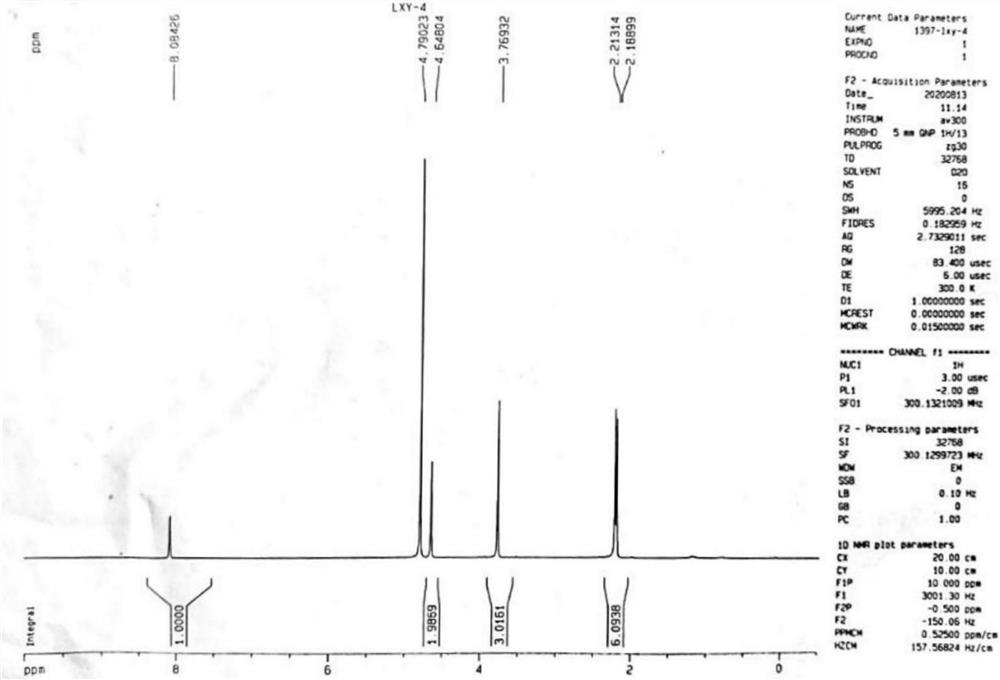
[0070] Take 1g of 3,5-dimethyl-4-methoxypyridine nitrogen oxide in a three-necked flask, add 0.2mL of chloroform and 0.5mL of water to dissolve, and slowly add 0.68mL of dimethyl sulfate dropwise. Under argon protection, react at 80°C for 3h.
[0071] Post-treatment: Cool the reaction solution obtained above to room temperature, add 4 mL of water and 4 mL of chloroform for extraction, combine the organic phases, spin dry, DCM:MeOH=20:1 (volume ratio) column chromatography to obtain impurity I. 1 H NMR (300MHz, CDCl 3 )δ7.46(s,2H),4.00(s,3H),2.05(s,6H).HRMS(ESI,M / Z):Calculated for[M-X] + :154.09,[M-X-H+Na] + :176.07, found: 154.0859, 176.0683.
Embodiment 3
[0072] The preparation of embodiment 3 impurity III
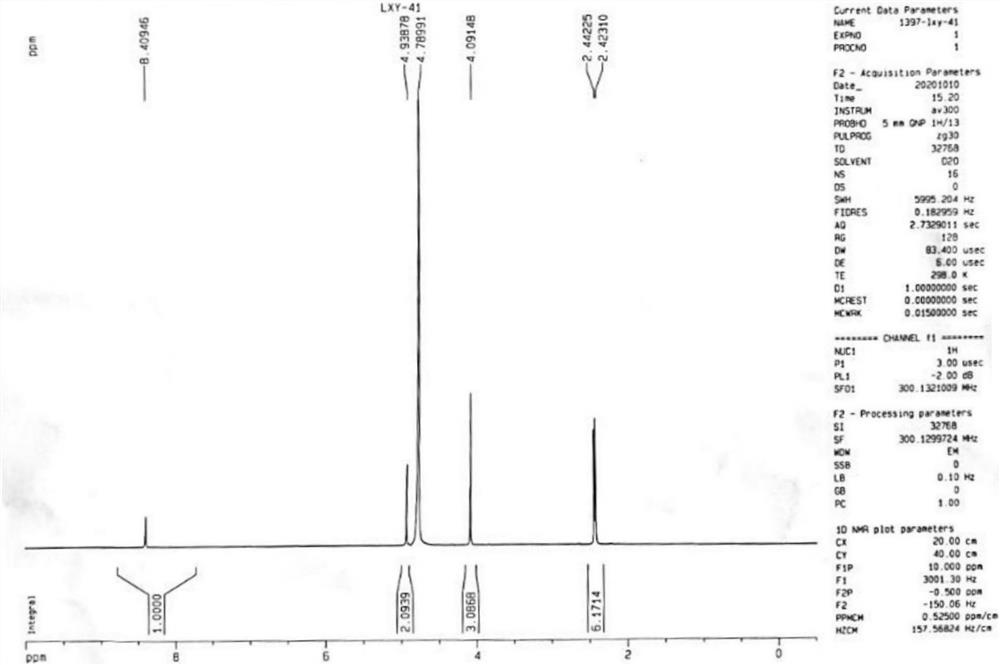
[0073]
[0074] ZrCl 4 (815mg, 3.5mmol) was dissolved in dry tetrahydrofuran (35mL), and NaBH 4 (500mg, 13.2mmol), after stirring for 10 minutes, the reaction solution A was obtained; 3,5-dimethyl-4-methoxypyridine nitrogen oxide (500mg, 3.3mmol) was dissolved in dry tetrahydrofuran (15mL) , to obtain a reaction solution B; add the reaction solution B to the reaction solution A at 0-5° C., rise to room temperature, and stir to react. The progress of the reaction was monitored by TLC. After the reaction was completed, it was cooled to 0°C, and dilute hydrochloric acid was added dropwise to quench the reaction. Tetrahydrofuran was distilled off under reduced pressure, the aqueous layer was extracted with ethyl acetate (30 mL×3 times), the organic layers were combined, washed with saturated brine, and dried over anhydrous sodium sulfate. After the organic phase was spin-dried, it was chromatographed with ethyl acetate / p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com