Decomposition method for analyzing trace impurities of metal organic compounds
A technology for organic compounds and trace impurities, applied in the field of chemical analysis, which can solve problems such as long decomposition reaction time, deviation of analysis results, and complicated hydrogen chloride gas preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
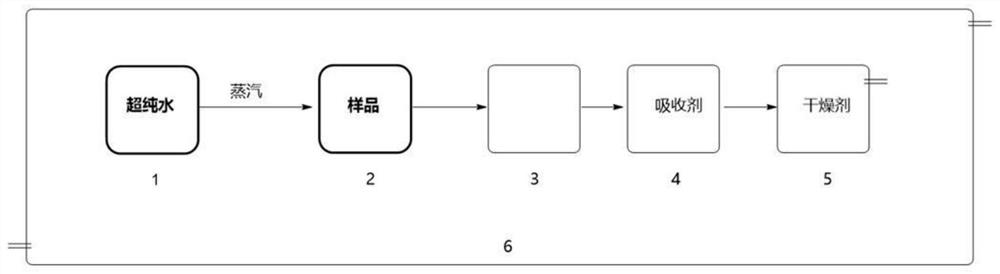
[0018] Put the dry sampling bottle and quartz dropper that have been accurately weighed into the inert atmosphere operation box. The oxygen content in the inert atmosphere operation box is maintained between 0.3 and 0.6ppm. in the bottle. The total weight of the sampling bottle and the metal-organic compound therein was accurately weighed on an analytical balance, so that the weight of the metal-organic compound in the sampling bottle was 0.1 g. Press as figure 1 The shown structure connects the sampling bottle, the buffer bottle, the absorbing bottle and the drying bottle in sequence. Water is housed in the absorption bottle as an absorbent, and the temperature of the water is maintained at 0°C. The desiccant bottle is filled with color-changing silica gel or calcium oxide desiccant. The buffer bottle is an empty bottle.
[0019] Heat the ultrapure water, and when more and stable water vapor is generated, then pass it into the sampling bottle 2 with a thin catheter, and a...
Embodiment 2
[0035] Put the dry sampling bottle and stainless steel sampling spoon that have been accurately weighed into an inert atmosphere operation box. The oxygen content in the inert atmosphere operation box is maintained between 0.3 and 0.6ppm. Use a stainless steel sampling spoon to take 0.1 g in the sampling bottle. The total weight of the sampling bottle and the metal-organic compound therein was accurately weighed on an analytical balance, so that the weight of the metal-organic compound in the sampling bottle was 0.1 g. Press as figure 1 The shown structure connects the sampling bottle, the buffer bottle, the absorbing bottle and the drying bottle in sequence. Wherein the absorption bottle is equipped with water as an absorbent, and the temperature of the water is kept at 20±5°C. The desiccant bottle is filled with color-changing silica gel or calcium oxide desiccant. The buffer bottle is an empty bottle.
[0036] Heat the ultrapure water, and when more and stable water vap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com