Method for detecting purity of pimpinolide
A technology for the purity of anislide, which is applied in the field of detection of the purity of anislide, can solve the problems of poor separation of compounds with similar properties, few reports on the detection of the purity of anislide, waste of manpower, material and financial resources, etc., to achieve Reliable test results, saving time and material costs, rapid test results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
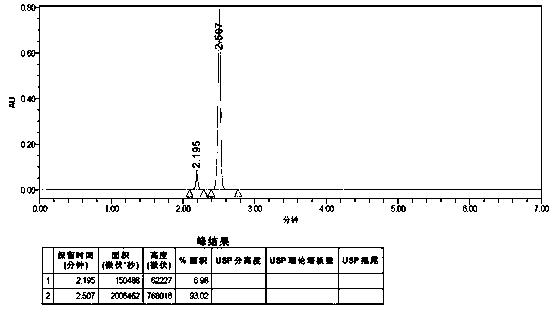
Embodiment 1
[0065] A method for detecting the purity of anisolactone, comprising detecting by ultra-high performance liquid chromatography, the conditions for the detection of ultra-high performance liquid chromatography are:
[0066] Chromatographic conditions:
[0067] Instrument: Waters ACQUITY UPLC H—Class;
[0068] Chromatographic column: reversed-phase C18 column (Yuexu Ultimate UHPLC AQ—C18, 1.8 μm, 2.1 * 100 mm, W—Port);
[0069] The mobile phase is mixed with acetonitrile and water, and chromatographically pure acetonitrile and water are used respectively; 0 min-2.88 min is elution, and after elution, the chromatographic column is washed and column balanced for subsequent use. This example is in 2.88 Min—5.00 min for column flushing, 5.00 min—7.00 min for column equilibrium elution, and column flushing. The specific elution solvents, flushing solvents, and equilibrium solvents used for elution, flushing, and equilibrium are operated according to Table 3;
[0070]
[0071] Co...
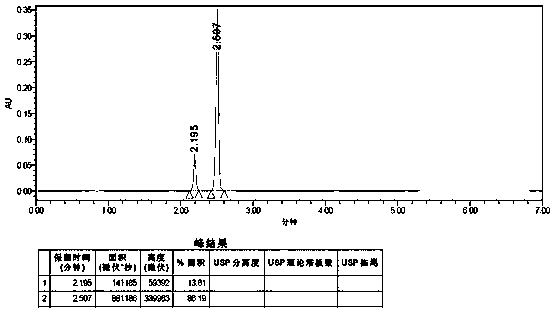
Embodiment 2
[0078] A method for detecting the purity of anisolactone, comprising detecting by ultra-high performance liquid chromatography, the conditions for the detection of ultra-high performance liquid chromatography are:
[0079] Chromatographic conditions:
[0080] Instrument: Waters ACQUITY UPLC H—Class;
[0081] Chromatographic column: reversed-phase C18 column (Yuexu Ultimate UHPLC AQ—C18, 1.8 μm, 2.1 * 100 mm, W—Port);
[0082] The mobile phase is mixed with acetonitrile and water, using chromatographically pure and pure water respectively; 0 min-2.88 min is elution, and after elution, the chromatographic column is washed and column balanced for subsequent use. This example is in 2.88 Min—5.00 min for column flushing, 5.00 min—7.00 min for column equilibrium elution, and column flushing. The elution solvent, flushing solvent, and equilibration solvent used for specific elution, flushing, and equilibrium are operated according to Table 4;
[0083]
[0084] Column temperature...
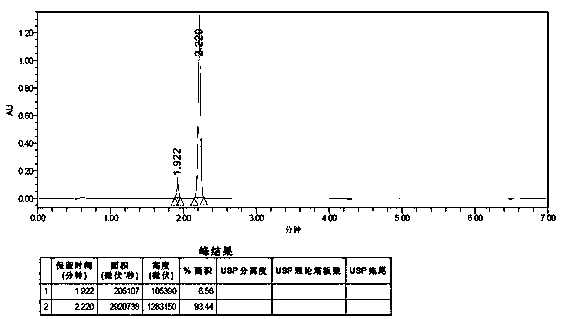
Embodiment 3
[0091] A method for detecting the purity of anisolactone, comprising detecting by ultra-high performance liquid chromatography, the conditions for the detection of ultra-high performance liquid chromatography are:
[0092] Chromatographic conditions:
[0093] Instrument: Waters ACQUITY UPLC H—Class;
[0094] Chromatographic column: reversed-phase C18 column (Yuexu Ultimate UHPLC AQ—C18, 1.8 μm, 2.1 * 100 mm, W—Port);
[0095] The mobile phase is a mixture of methanol and water, using chromatographically pure and pure water respectively; 0 min-2.88 min is elution, and after elution, the chromatographic column is washed and column balanced for subsequent use. This example is in 2.88 Min—3.60 min for column flushing, 5.00 min—7.00 min for column equilibration elution and column flushing, the specific elution solvent, flushing solvent, and equilibration solvent used for elution, flushing, and equilibration are operated according to Table 5;
[0096]
[0097] Column temperatur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Column length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com