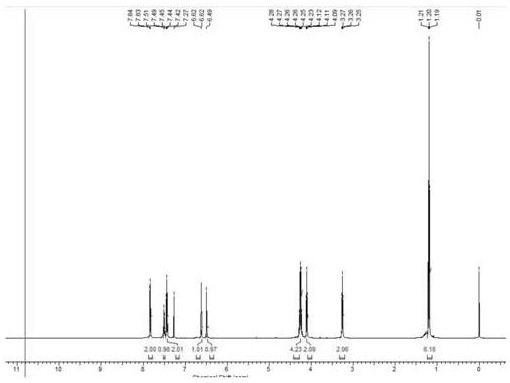
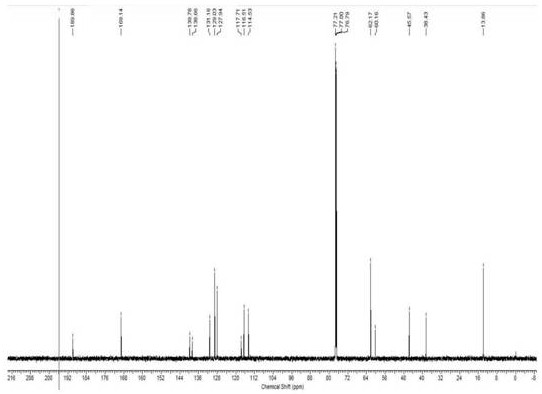
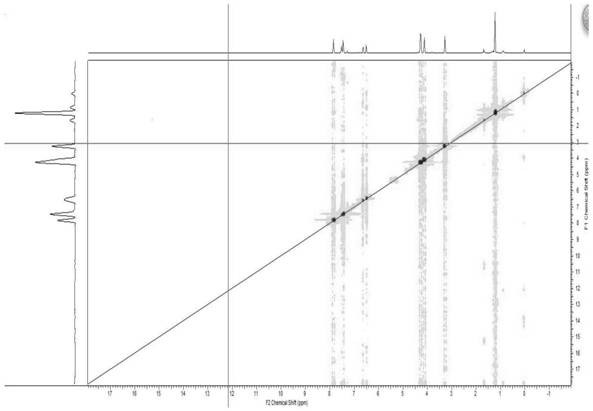
Ketorolac impurity C and preparation method and application thereof
A technology for ketorolac and impurities, which is applied in the field of preparation of ketorolac impurities and achieves the effects of less by-products, good reproducibility and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of Compound 3
[0032]
[0033] Pyrrol (10.0 g, 149 mmol), tetrabutylammonium sulfate (5.0 g, 14.9 mmol) was added to the reaction bottle, and 200 mlNOH 50% solution was added: in a mixed solution of dichloromethane (1: 2.5V / v), 0 Benzulfonyl chloride (29.0 g, 164 mmol) was slowly added dropwise, and 15 min was added dropwise, and 200 ml of dichloromethane was added to the room temperature. The organic phase was washed with water, and the filtrate was dried. EtOAc EtOAc EtOAc EtOAc EtOAc EtOAc EtOAc.
Embodiment 2
[0034] Example 2: Preparation of Compound 4
[0035]
[0036] Anhydrous aluminum chloride (20.0 g, 150 mmol) was dissolved in 100 mL of dichloroethane, and then stirred slowly with benzoyl chloride (21.0 g, 150 mmol), stirred at room temperature for 10 min, and finally added Compound 3 (25.0 g, 120 mmol), at room temperature 1H; 200 mL of dichloromethane, phase phase, washed with water, washed with 10% NaHCO3, dry NASO4, filtered, concentrated filtrate, obtained white solid is Compound 4 36.0g, yield 95.8%.
Embodiment 3
[0037] Example 3 Preparation of Compound 5
[0038]
[0039] Compound 4 (35.0 g, 112 mmol), sodium hydroxide (18.0 g, 450 mmol) was added to the reaction flask, and then 200 ml of dioxane: water (4: 1V / V) mixed solvent was added, and the reaction was stirred at room temperature for 12 h. 200 ml of ethyl acetate was added, phased, and the organic phase was washed with water, waterless NASO4 dried, filtered, concentrated filtrate, the obtained white solid was compound 5, 18.0 g, yield 93.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com