Sweetening agent for cigarettes as well as preparation method and application of sweetening agent
A technology for sweeteners and cigarettes, applied in the application and preparation of tobacco, tobacco and other directions, can solve problems such as poor stability, easy deterioration and damage, and achieve the effects of softening smoke, improving aroma quality, and increasing oral sweetness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
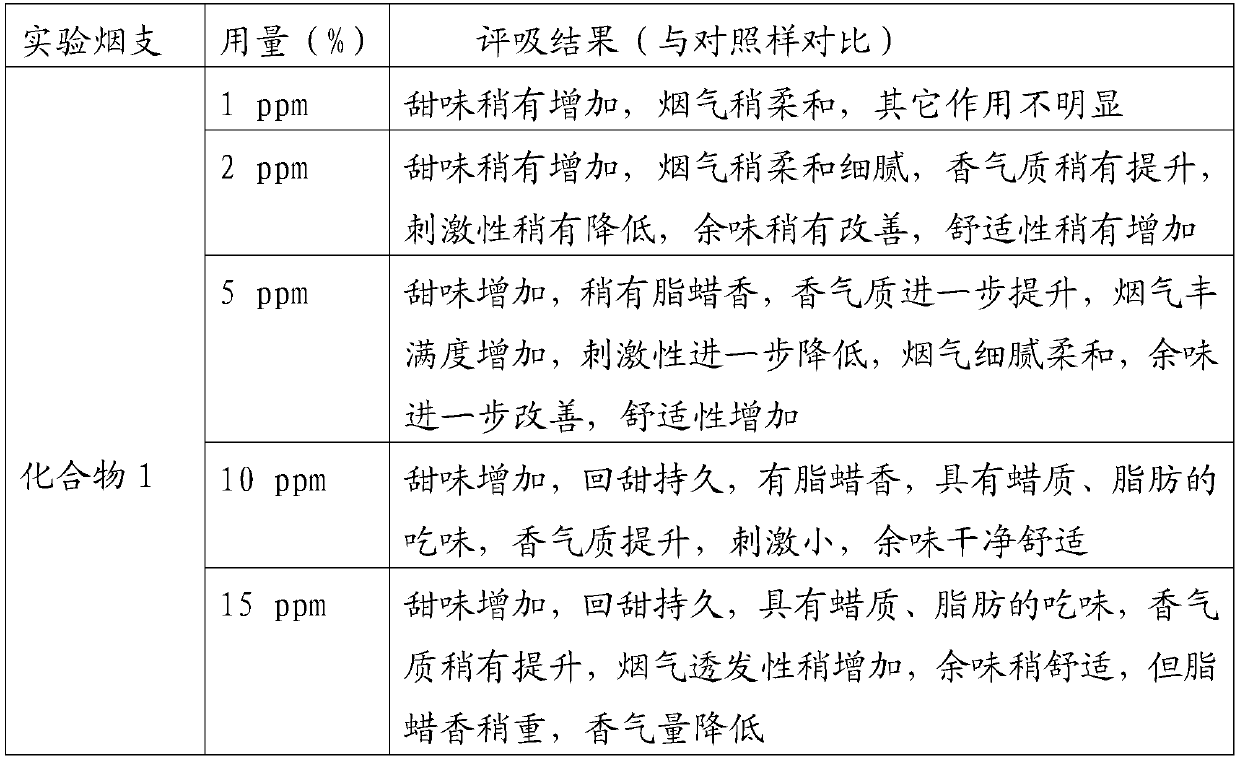
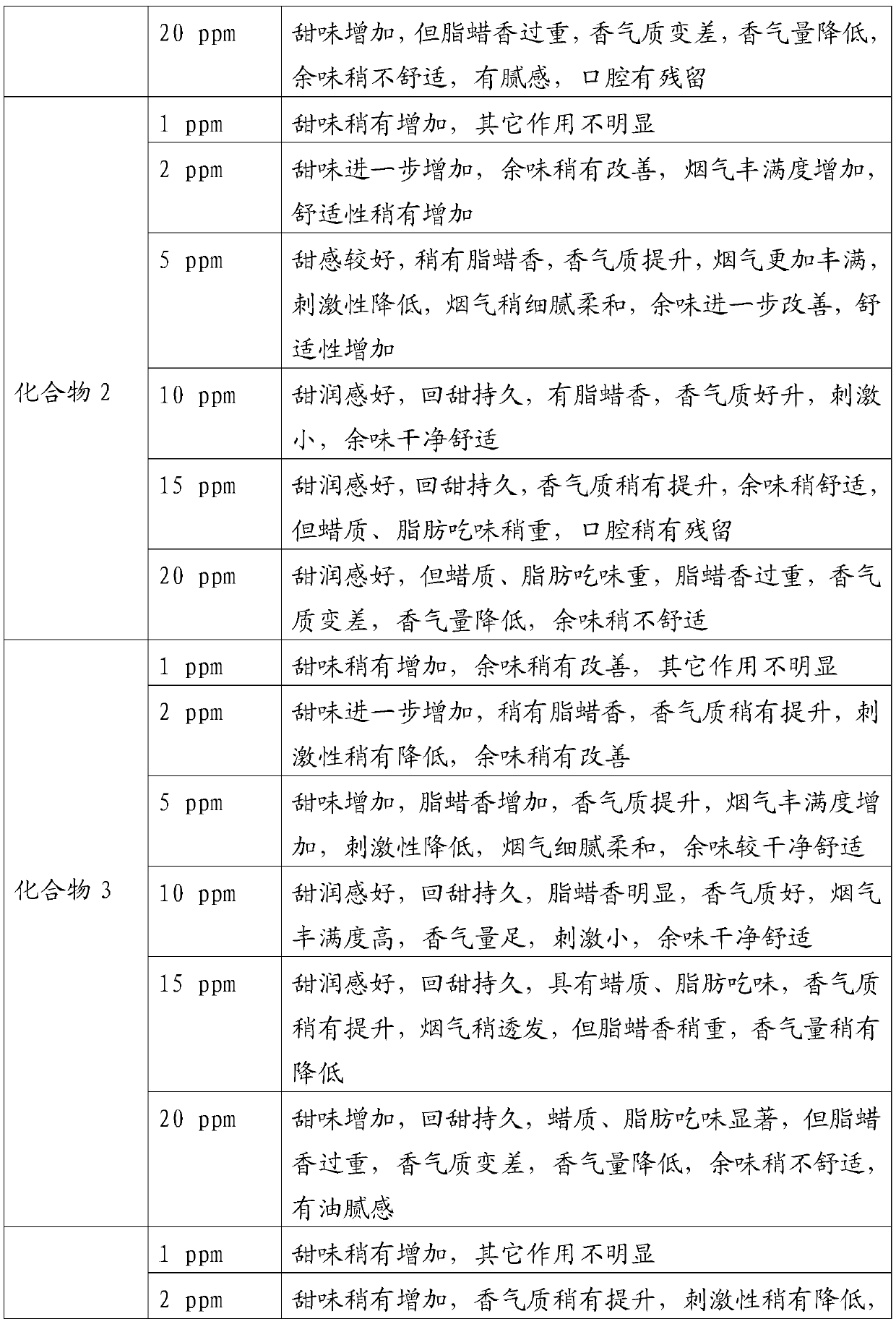
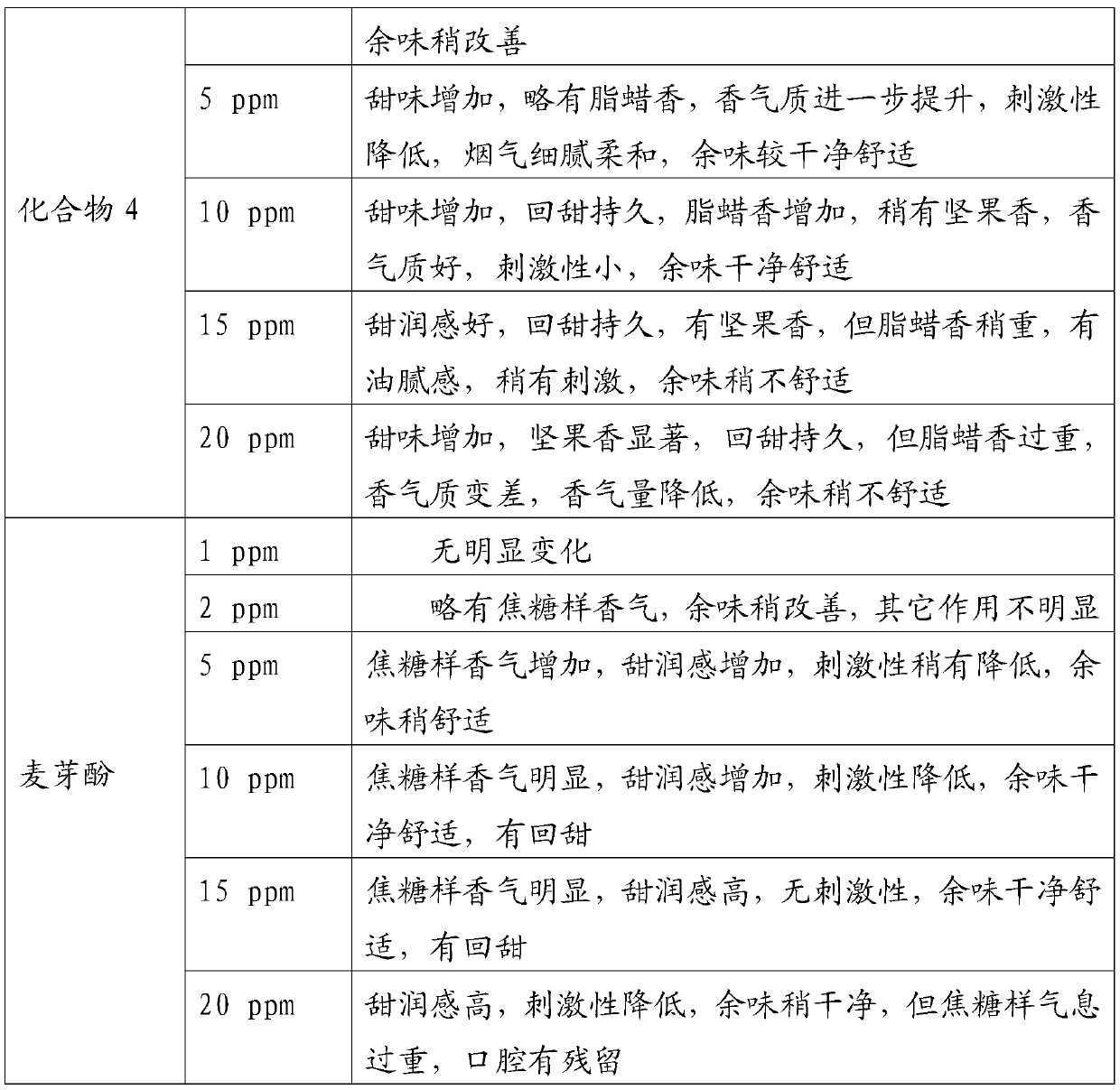
Examples
preparation example Construction
[0029] The preparation method of above-mentioned sweetener for tobacco is:
[0030] Its M is an acylating reagent, specifically
[0031] said It is one of lauroyl chloride, myristoyl chloride, palmitoyl chloride or stearyl chloride;
[0032] said It is one of lauric anhydride, myristic anhydride, palmitic anhydride or stearic anhydride.
Embodiment 1
[0034] In a 100mL round bottom flask, add 2.88g 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (20mmol) and 50mL anhydrous dichloromethane, cool To 0 ℃, under the protection of nitrogen, add 4.44g triethylamine (44mmol) and 9.84g lauroyl chloride (45mmol) successively, then rise to room temperature and react for 12h, after the reaction is completed, the reaction solution is washed with water and saturated sodium chloride solution for 3 Second, the organic phase is dried with anhydrous sodium sulfate, and the solvent is evaporated under reduced pressure (in this embodiment and the following embodiments, the vacuum degree of reduced pressure is determined according to actual conditions and needs, as long as the effect of evaporation under reduced pressure is realized. Yes, it will not affect the realization of the technical solution due to the difference in vacuum degree), the crude product was separated and purified by silica gel column chromatography, and petroleum ether (V)...
Embodiment 2
[0036] In a 100mL round bottom flask, add 2.88g of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (20mmol) and 50mL of anhydrous toluene, and cool to - 5°C, under the protection of nitrogen, add 3.48g pyridine (44mmol) and 17.22g acetic anhydride (45mmol) successively, then rise to room temperature and react for 10h. The phase was dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The crude product was separated and purified by silica gel column chromatography, eluting with petroleum ether (V): ethyl acetate (V) = 8:1 to obtain 8.02 g of light yellow oil , is 2,3-dihydro-3,5-dilauroyloxy-6-methyl-4H-pyran-4-one, and the yield is 78.94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com