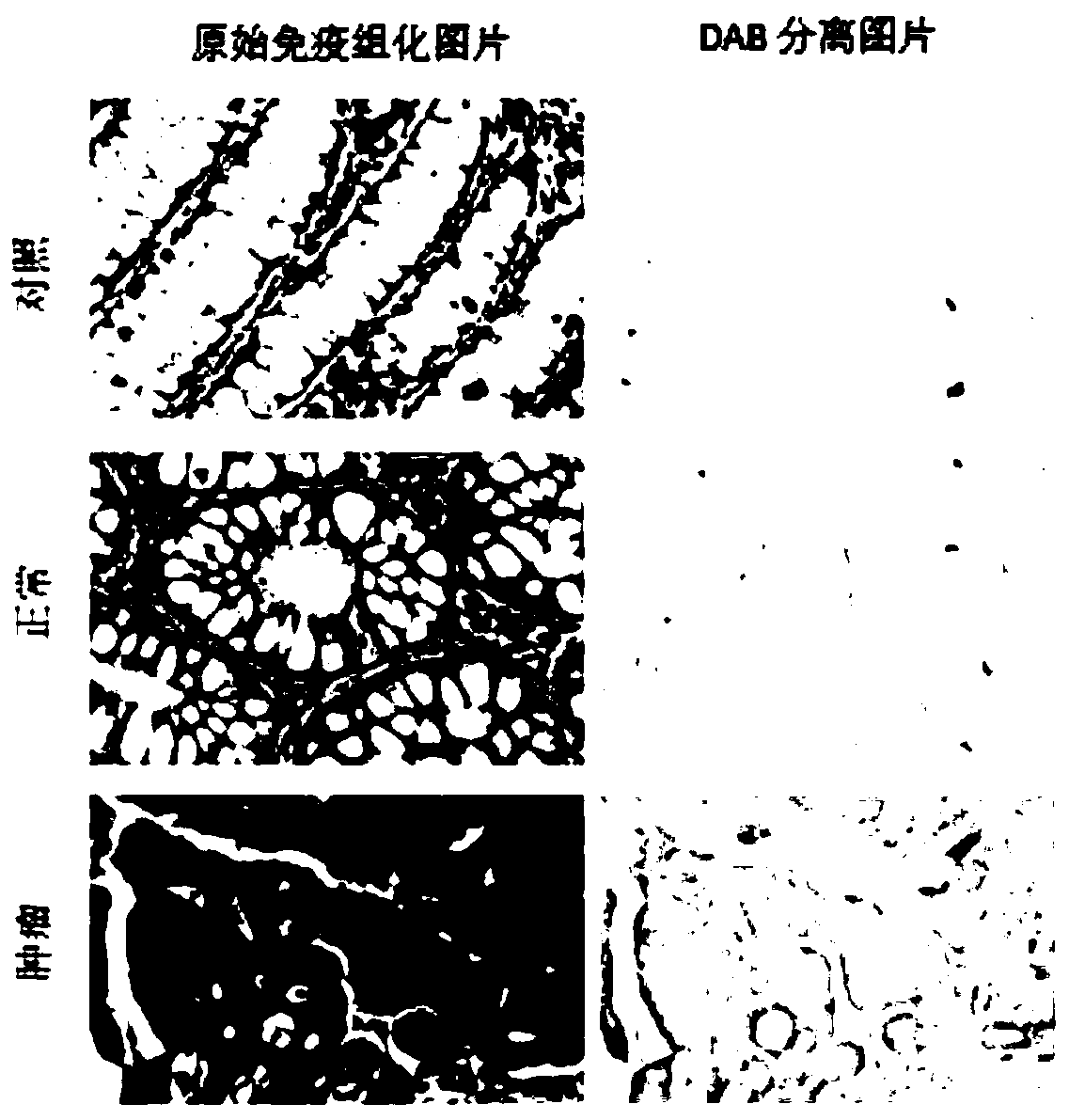
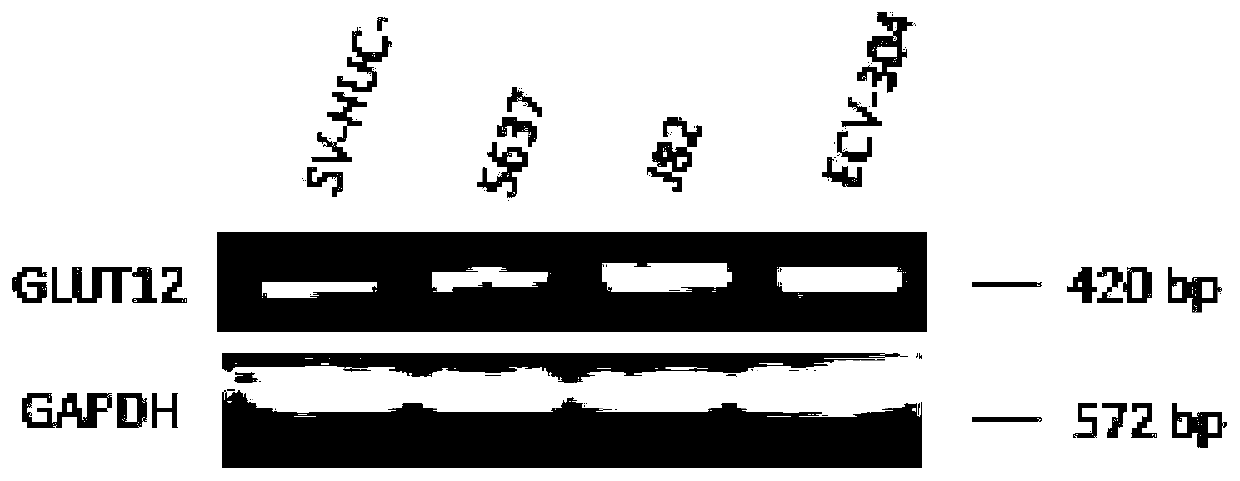GLUT12 protein inhibitor and preparation method and application thereof
A protein inhibitor, injection technology, applied in the field of medicine, can solve the problem that the expression of GLUT12 is not very clear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 The GLUT12 protein inhibitor of the present invention was prepared with the following components: 0.5% cisplatin, 3% curcumin, 3% lecithin for injection, 5% soybean oil for injection, 0.1% sodium sulfite, and 20% glycerin , Tween-809%, water for injection is added to 100% mass ratio;
[0051] (1) Preparation of the oil phase: add lecithin for injection and Tween-80 to the soybean oil for injection respectively, stir to dissolve it, and use it as the oil phase;
[0052] (2) Preparation of colostrum: Add cisplatin, curcumin and sodium sulfite into water, stir to dissolve them, then add the oil phase prepared in (1) into water, and disperse at high speed shear (speed: 15000 / min, dispersion time :30min), form colostrum;
[0053] (3) High-pressure homogenization: adjusting step (2) to obtain pH 7.2 of the colostrum, and high-pressure homogenization to obtain essence milk;
[0054] (4) Sterile filtration: the refined milk is sterilized by filtration through a 0.22...
Embodiment 2
[0055] Example 2 The GLUT12 protein inhibitor of the present invention was prepared with the following components: cisplatin 0.1%, curcumin 1%, lecithin for injection 1%, soybean oil for injection 35%, sodium sulfite 0.2%, glycerol 15% , Tween-805%, water for injection is added to 100% mass ratio;
[0056] (1) Preparation of the oil phase: add lecithin for injection and Tween-80 to the soybean oil for injection respectively, stir to dissolve it, and use it as the oil phase;
[0057] (2) Preparation of colostrum: Add cisplatin, curcumin and sodium sulfite into water, stir to dissolve them, then add the oil phase prepared in (1) into water, and disperse at high speed shear (speed: 15000 / min, dispersion time :30min), form colostrum;
[0058] (3) High-pressure homogenization: adjusting the pH of the colostrum obtained in step (2) to be 7.2, and high-pressure homogenization to obtain essence milk;
[0059] (4) Sterile filtration: the refined milk is sterilized by filtration throu...
Embodiment 3
[0060] Example 3 The GLUT12 protein inhibitor of the present invention was prepared with the following components: cisplatin 0.2%, curcumin 2%, lecithin for injection 2%, soybean oil for injection 25%, sodium sulfite 0.3%, glycerol 18% , Tween-806%, water for injection is added to 100% mass ratio;
[0061] (1) Preparation of the oil phase: add lecithin for injection and Tween-80 to the soybean oil for injection respectively, stir to dissolve it, and use it as the oil phase;
[0062] (2) Preparation of colostrum: Add cisplatin, curcumin and sodium sulfite into water, stir to dissolve them, then add the oil phase prepared in (1) into water, and disperse at high speed shear (speed: 15000 / min, dispersion time :30min), form colostrum;
[0063] (3) High-pressure homogenization: adjusting the pH of the colostrum obtained in step (2) to be 7.0, and high-pressure homogenization to obtain essence milk;
[0064] (4) Sterile filtration: the refined milk is sterilized by filtration through...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com