Method for recycling tin from tin-containing lead slag
A technology of tin lead and tin chloride, applied in the field of solid waste recycling, can solve problems such as environmental pollution and resource waste, and achieve the effect of simple and controllable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
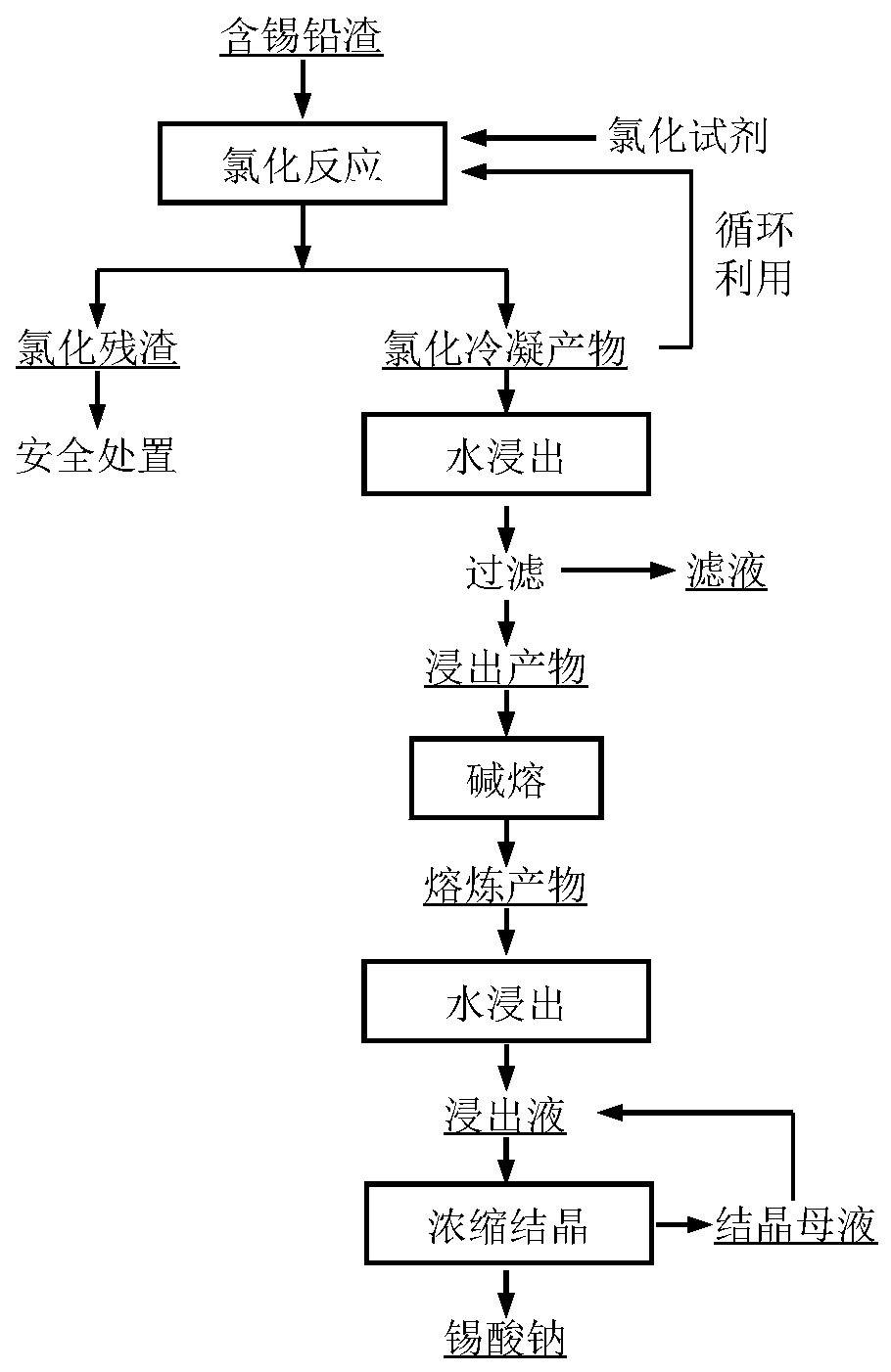
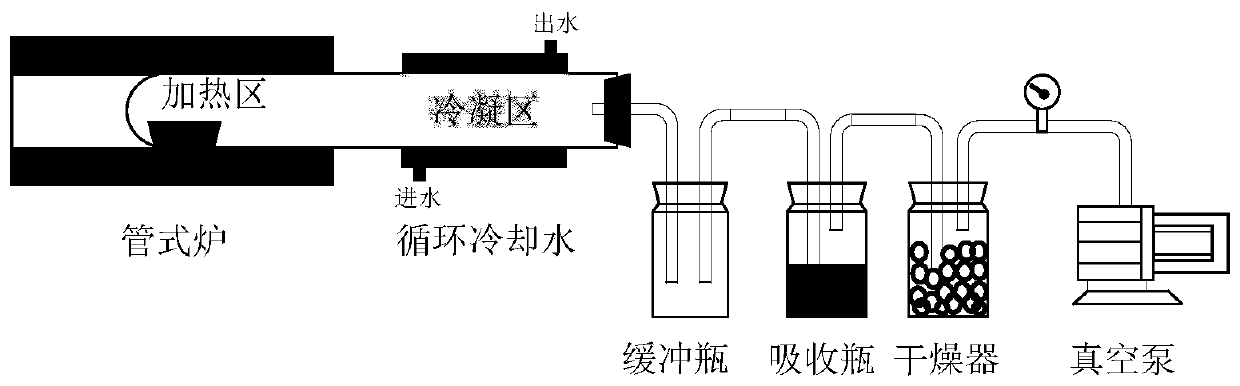
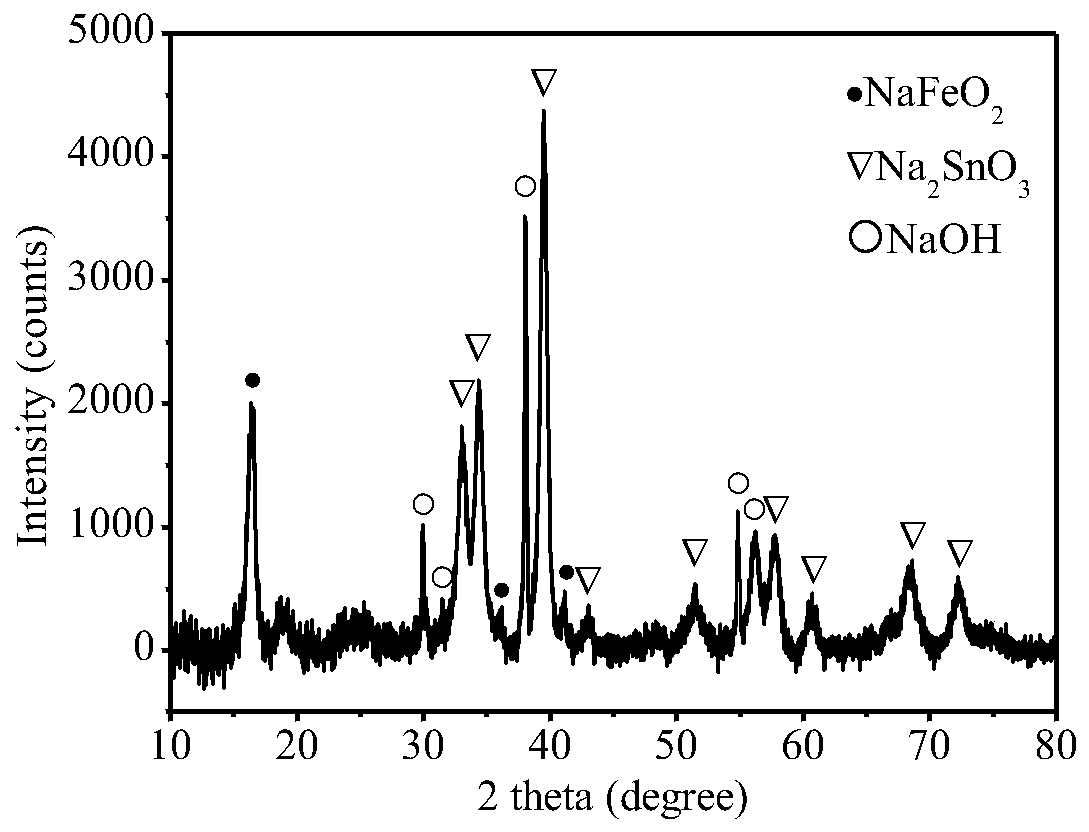
[0057] Evenly mix 1g of tin-lead slag and 5g of ammonium chloride, press the mixture into a block, place it in a crucible, place the crucible in the heating zone of the quartz tube, control the heating temperature to 500°C, and the heating time to 30min. Adjust so that the air pressure in the system is 100kPa. After the chlorination reaction is finished, the remaining residue in the crucible is chlorination residue, and the chlorination condensation product is obtained in the condensation zone. The tin content in the chlorinated condensate was 0.73 wt%. The chlorination condensed product is subjected to water leaching, the leaching solid-to-liquid ratio is controlled to be 100g / L, and the leaching time is 2h. After the leaching is completed, the solid and liquid are separated to obtain the leaching product and filtrate. The dried leaching product is mixed with sodium hydroxide according to the mass ratio of 1:6, and the alkali fusion reaction is carried out. The alkali fusion...
Embodiment 2
[0059] Evenly mix 1g of tin-lead slag with 30g of ammonium chloride, press the mixture into a block, place it in a crucible, place the crucible in the heating zone of the quartz tube, control the heating temperature to 700°C, and the heating time to 90min. Adjust so that the air pressure in the system is 5 Pa. After the chlorination reaction is completed, the remaining residue in the crucible is chlorination residue, and the chlorination condensation product is obtained in the condensation zone. Mix the obtained chlorination condensate with tin-lead slag again, press it into a block, place it in a crucible for another chlorination reaction, control the chlorination conditions to be the same as the first chlorination, and obtain chlorination after the reaction is over. The condensed product was recirculated twice to obtain the chlorinated condensed product. The tin content in the chlorinated condensate was 2.36 wt%. The chlorinated condensation product is subjected to water le...
Embodiment 3
[0061] Evenly mix 1g of tin-lead slag with 15g of ammonium chloride, press the mixture into a block, place it in a crucible, place the crucible in the heating zone of the quartz tube, control the heating temperature to 350°C, and the heating time to 15min. Adjust so that the air pressure in the system is 50 Pa. After the chlorination reaction is finished, the remaining residue in the crucible is chlorination residue, and the chlorination condensation product is obtained in the condensation zone. The tin content in the chlorinated condensate was 0.52 wt%. The chlorination condensed product is subjected to water leaching, the leaching solid-to-liquid ratio is controlled to be 50g / L, and the leaching time is 0.5h. After the leaching is completed, the solid and liquid are separated to obtain the leaching product and filtrate. The dried leaching product is mixed with sodium hydroxide at a mass ratio of 1:3 for alkali fusion reaction, the alkali fusion temperature is controlled at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com