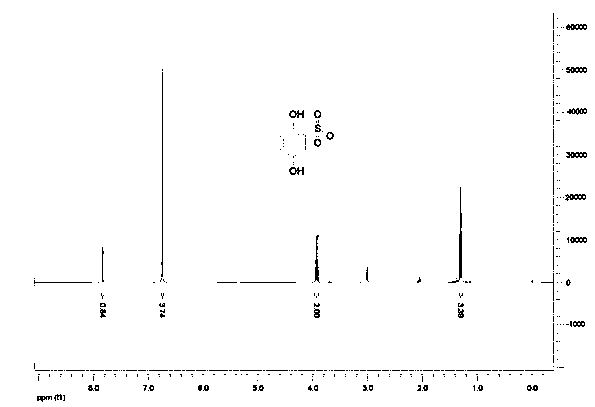
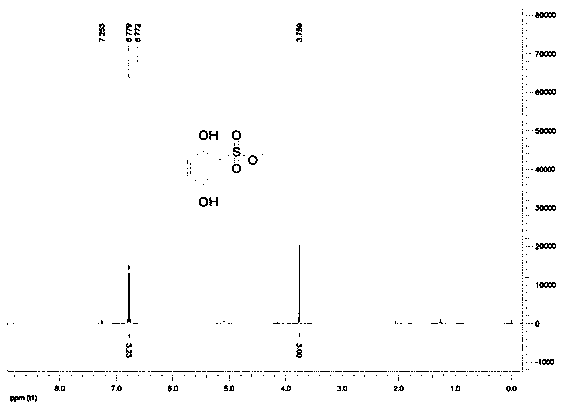
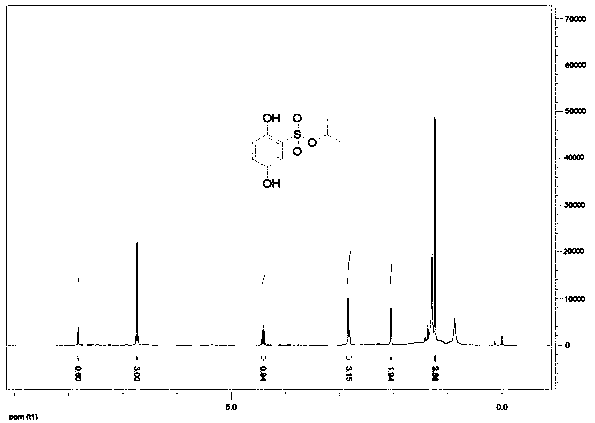
Synthesis process of hydroxybenzene sulfonate compound
A technology of dobesilate and synthesis process, which is applied in the preparation of sulfonate and organic chemistry, and can solve the problems of structure and synthesis method that have not been reported in literature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, the preparation of methyl dobesylate
[0031] Add 400g of methanol, 200g of hydroxybenzenesulfonic acid, and 18g of concentrated sulfuric acid into a 1000ml four-necked reaction flask, start stirring and heating, and raise the temperature to 60-70°C for 12 hours. After the reaction was completed, excess methanol was evaporated under reduced pressure, 400g of water was added, extracted 4 times with ethyl acetate (200ml / time), the combined organic phase was washed with 100ml of saturated edible salt water, and the organic phase was then rotary evaporated to recover the solvent, and the resulting yellow-brown The residue was separated by staged column chromatography with developing solvents ethyl acetate and petroleum ether in different proportions to obtain 20 g of ethyl dobesilate with higher purity.
Embodiment 2
[0032] Embodiment 2, the preparation of ethyl dobesylate
[0033] Add 600g of ethanol, 100g of dobensulfonic acid, and 9g of concentrated sulfuric acid into a 1000ml four-necked reaction flask, start stirring and heating, and raise the temperature to 75-85°C for 18 hours. After the reaction was completed, excess ethanol was evaporated under reduced pressure, 400g of water was added, extracted 4 times with ethyl acetate (200ml / time), the combined organic phase was washed with 100ml of saturated salt water, and the organic phase was rotary evaporated to recover the solvent, and the resulting yellow-brown The residue was separated by staged column chromatography with developing solvents ethyl acetate and petroleum ether in different proportions to obtain 17 g of ethyl dobesilate with higher purity.
Embodiment 3
[0034] Embodiment 3, the preparation of isopropyl dobesilate
[0035] Add 800g of isopropanol, 200g of hydroxybenzenesulfonic acid, and 9g of concentrated sulfuric acid into a 1000ml four-necked reaction flask, start stirring and heating, and raise the temperature to 80-90°C for 20 hours. After the reaction was completed, excess isopropanol was evaporated under reduced pressure, 400g of water was added, extracted 4 times with ethyl acetate (200ml / time), the combined organic phase was washed with 100ml of saturated edible salt water, and the organic phase was rotary evaporated to recover the solvent, and the obtained The yellow-brown residue was separated by column chromatography in different proportions with developing solvents ethyl acetate and petroleum ether to obtain 8 g of refined ethyl dobesilate with higher purity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com