Benzazepine derivatives
A technology of benzoazepines and benzodiazepines, which is applied in the field of benzoazepine derivatives and their salts, can solve problems such as ignorance, and achieve the effect of favorable metabolic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0087] [chemical formula 2]
[0088]
[0089] where the wavy line is the binding point, and A 1 and A 2 Same as defined above.
[0090] The present invention includes the embodiments shown below.
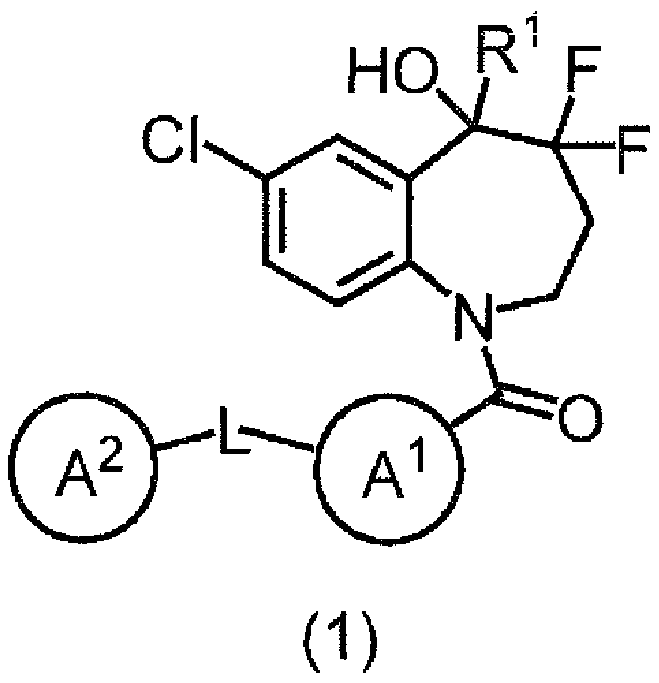
[0091] Item 1. A benzazepine compound of formula (1) or a salt thereof,
[0092] [chemical formula 3]
[0093]
[0094] where R 1 is deuterium, OH, COOH, optionally substituted C 1-6 Alkyl, optionally substituted C 1-6 Alkyl-O-CO- or optionally substituted C 2-6 Alkenyl;
[0095] L is a direct bond or -C(=O)-NH-;
[0096] Ring A 1 is a hydrocarbon ring or a heterocycle;
[0097] Ring A 2 is a hydrocarbon ring or a heterocycle; and
[0098] Ring A 1 and A 2 Each of may have at least one substituent.
[0099] Item 2. The compound or salt thereof according to Item 1, wherein Ring A 1 is a saturated or unsaturated 3 to 8 membered monocyclic hydrocarbon ring, or a saturated or unsaturated 3 to 15 membered monocyclic, bicyclic or tricyclic ring containing 1 to 5 het...
example
[0214] The present invention is described in detail in the following Reference Examples, Examples and Test Examples, but the present invention is not limited thereto. These examples may be modified without departing from the scope of the invention.
[0215] The following abbreviations may be used herein.
[0216] REX: Reference instance number
[0217] EX: instance number
[0218] STR: Structural formula (In the formula, a structure with "chirality" refers to the absolute configuration.)
[0219] RProp: Preparation method (The product was prepared according to the method described in the referenced example with number, using the corresponding starting material.)
[0220] Prop: Preparation method (The product was prepared according to the method described in the numbered examples using the corresponding starting materials.)
[0221] Data: Physical data (NMR1: 1 δ(ppm) in H-NMR (dimethyl sulfoxide-d 6 ); NMR2: 1 δ(ppm)(CDCl in H-NMR 3 ); NMR3: 1 δ(ppm)(CD in H-NMR 3 OD...
example 1
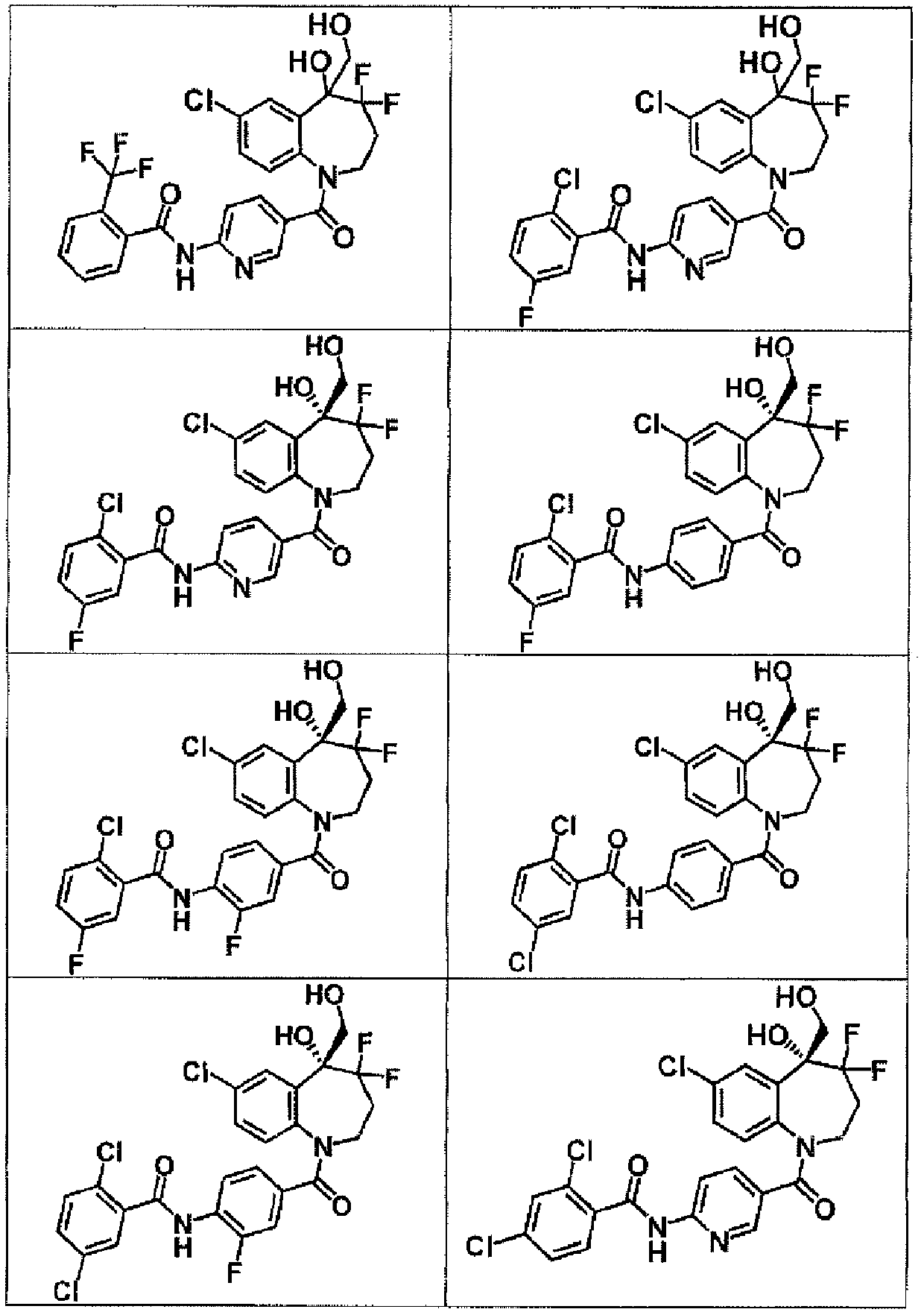
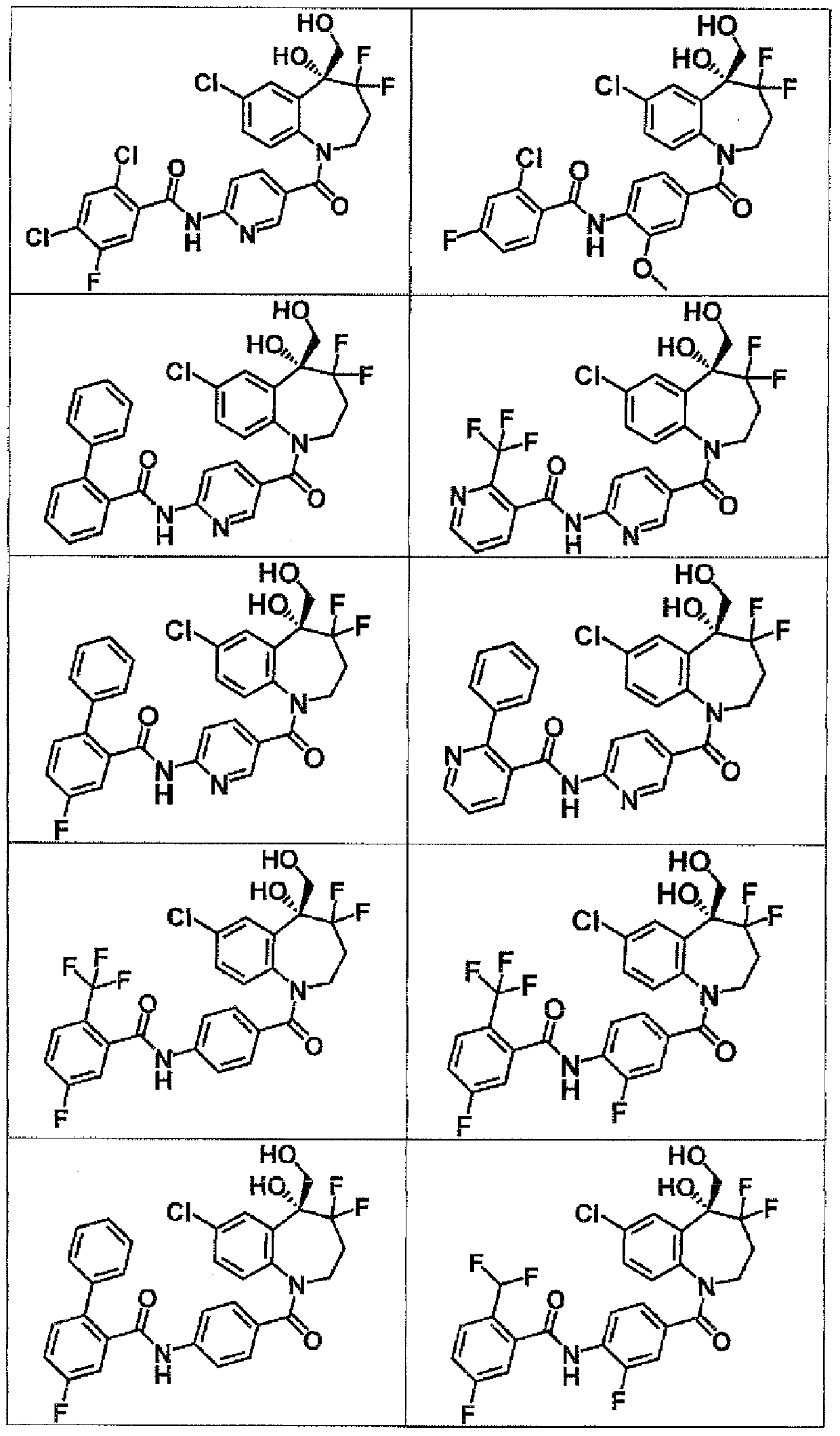
[0517] To a solution of 6-(2-chlorobenzamido)pyridine-3-carboxylic acid (113 mg) in DMA (1.0 mL) was added SOCl under ice cooling under nitrogen atmosphere 2 (30.0 μL), and the mixture was stirred at the same temperature for 2 hours. To this was added 7-chloro-4,4-difluoro-5-(hydroxymethyl)-2,3,4,5-tetrahydro-1H-1-benzazepin-5-ol at the same temperature (90.0 mg), and the mixture was stirred at room temperature for 3 days. Add saturated NaHCO 3 Aqueous solution and water were added to the reaction solution, and the precipitate was filtered and purified by basic silica gel column chromatography (hexane / AcOEt) to give 2-chloro-N-{5-[7-chloro-4,4-di Fluoro-5-hydroxy-5-(hydroxymethyl)-2,3,4,5-tetrahydro-1H-1-benzazepine-1-carbonyl]pyridin-2-yl}benzamide (50.0 mg).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com