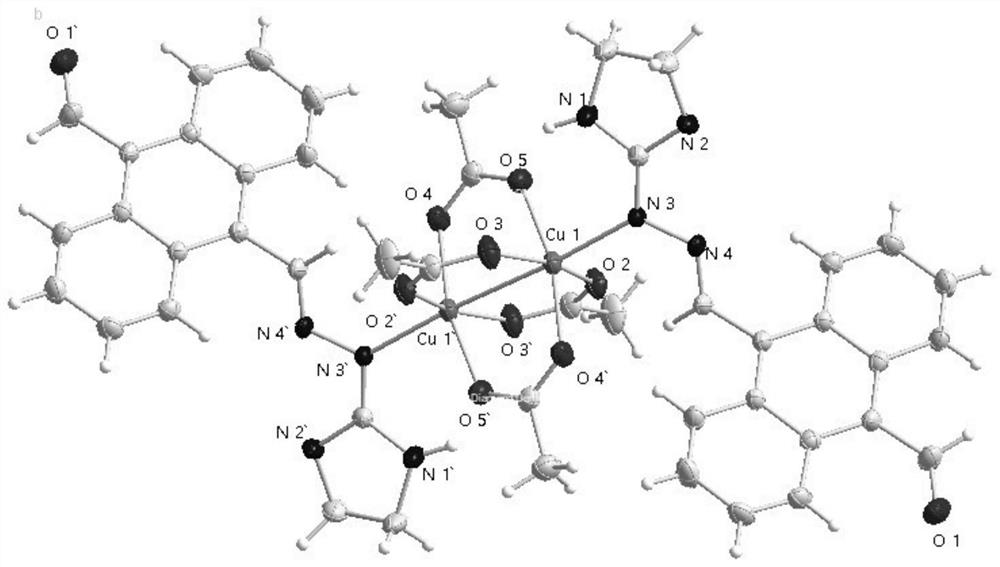
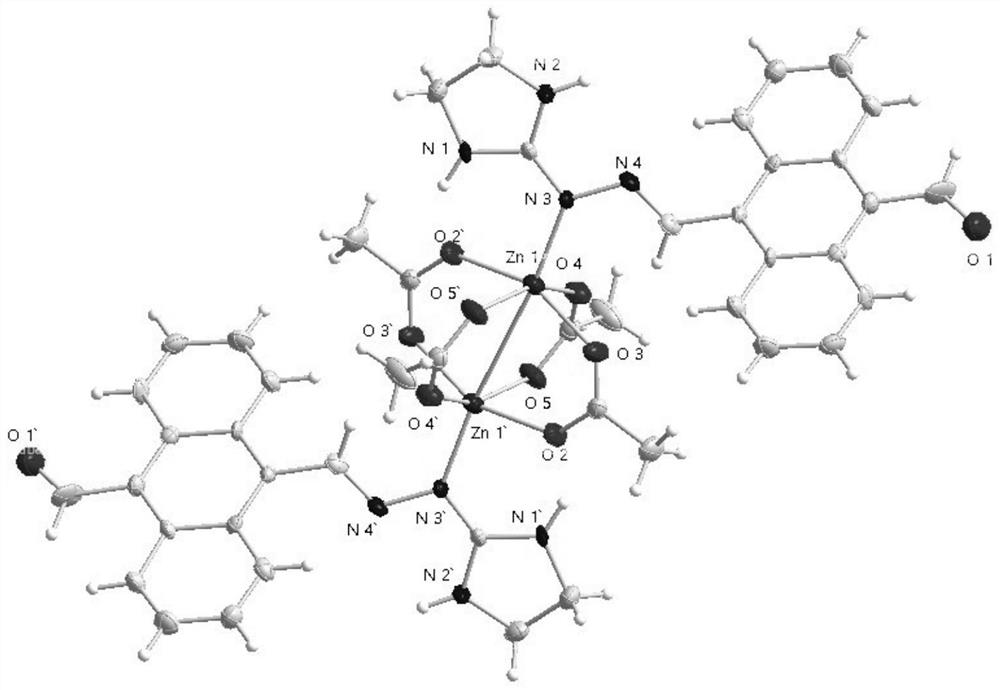
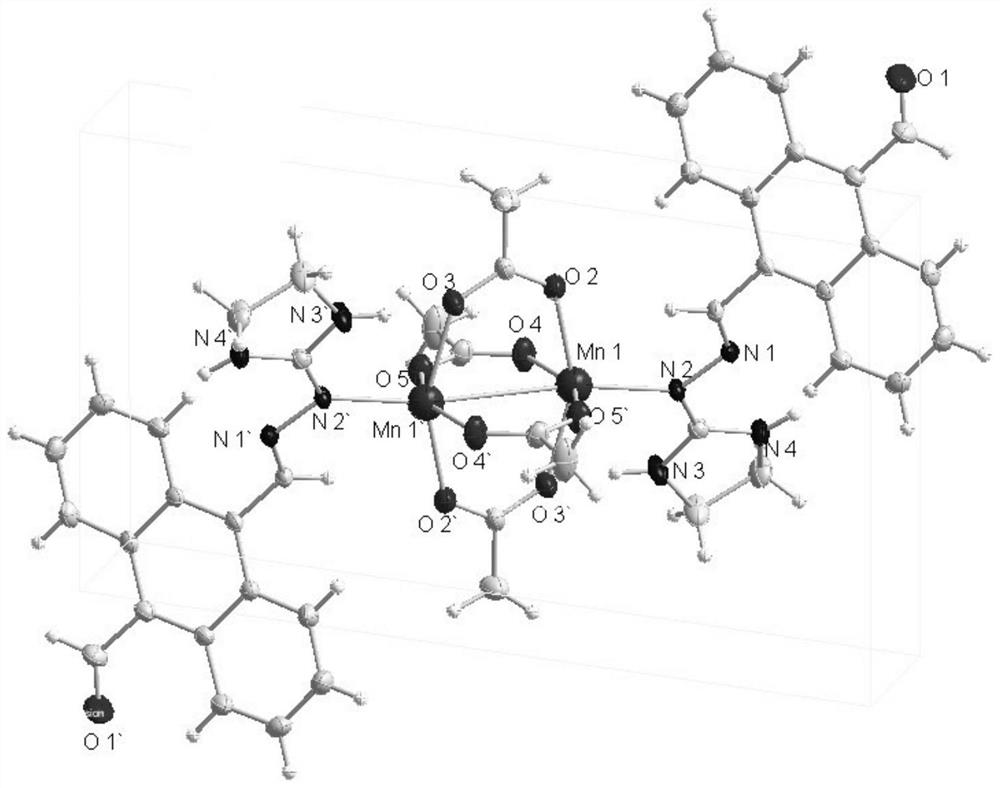
Binuclear metal complex with 9-formyl-10-imidanthracene as ligand, its synthesis method and application
A synthesis method and compound technology, applied in the field of medicine, can solve problems such as the synthesis method and application of binuclear metal complexes that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Synthesis of 9-formyl-10-imanthrenehydrazone-binuclear copper (II) complex (hereinafter also referred to as copper complex)
[0032] 0.15mmol of 9-formyl-10-imidanthracene and 0.20mmol Cu(Ac) 2 ·H 2 O was dissolved in a polar solvent composed of 10.0mL methanol and 8.0mL chloroform, and reacted at 50°C (reflux for condensation) for 4 hours. After the reaction, the solution was cooled to obtain a brown suspension, and a large amount of complex solids were formed; filtered and collected filter cake to obtain a brown powdery solid product. The product was recrystallized from methanol to obtain a brown square block single crystal with a yield of 50%.
[0033] The brown powdery solid product obtained above was characterized by infrared spectroscopy, elemental analysis and electrospray mass spectrometry, and the specific spectral characteristic data are as follows:
[0034] Infrared spectrum: (KBr,cm -1 ) 3332, 2925, 1661, 1618, 1432, 1390, 1286, 1050, 753, 601...
Embodiment 2
[0045] Embodiment 2: the synthesis of copper complex
[0046] 0.25mmol of 9-formyl-10-imidanthrene hydrazone and 0.25mmol Cu(Ac) 2 ·H 2 O was dissolved in a polar solvent composed of 5mL ethanol and 20mL dichloromethane, and reacted at 60°C (reflux for condensation) for 6 hours. After the reaction, the solution was cooled to obtain a brown suspension, filtered, and the filter cake was collected to obtain a brown solid powder. , yield 45%.
[0047] The product obtained in this example was analyzed by infrared spectroscopy, elemental analysis and electrospray mass spectrometry, and it was determined that the obtained brown solid powder was the copper complex of the target compound.
Embodiment 3
[0048] Embodiment 3: the synthesis of copper complex
[0049] 0.20mmol of 9-formyl-10-imidanthracene and 0.25mmol Cu(Ac) 2 ·H 2 O was dissolved in a polar solvent consisting of 10.0mL methanol and 10.0mL chloroform, and reacted at 60°C (reflux for condensation) for 6 hours. After the reaction, the solution was cooled to obtain a brown suspension, filtered, and the filter cake was collected to obtain a brown solid powder. , yield 40%.
[0050] The product obtained in this example was analyzed by infrared spectroscopy, elemental analysis and electrospray mass spectrometry, and it was determined that the obtained brown solid powder was the copper complex of the target compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com