Method for simply synthesizing CsAgCl<2> pure-phase inorganic lead-free perovskite
An inorganic non-phase-pure technology, applied in the direction of inorganic chemistry, chemical instruments and methods, lead compounds, etc., can solve the problems of unfavorable large-scale production, complicated operation, and long time consumption, and achieve easy operation, enhanced fluorescence efficiency, and low energy consumption. small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
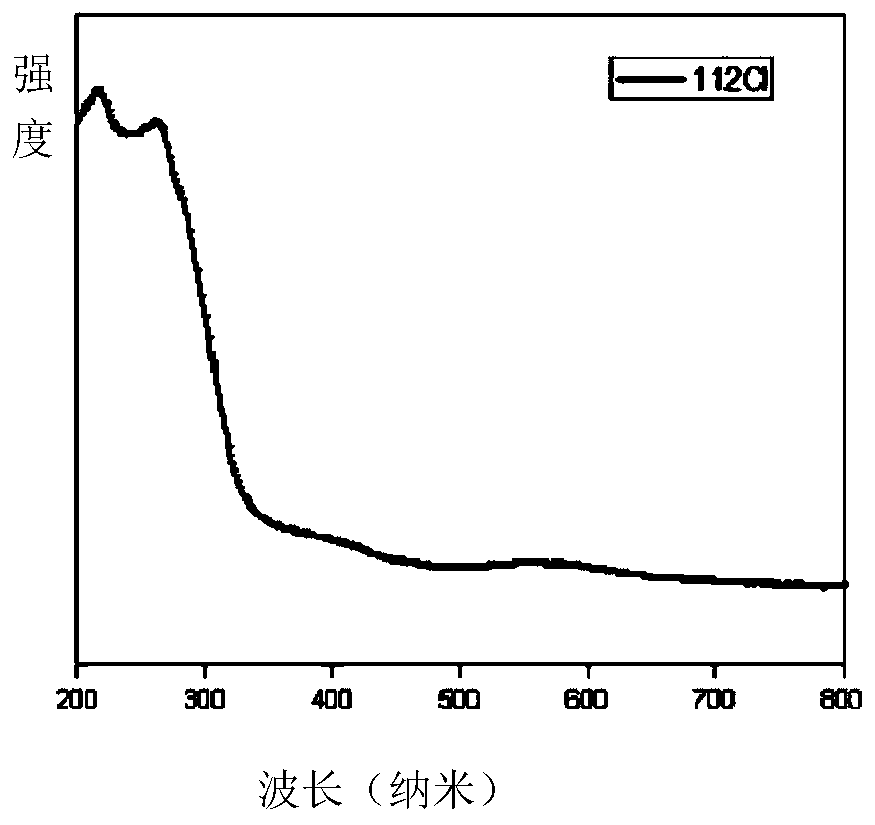
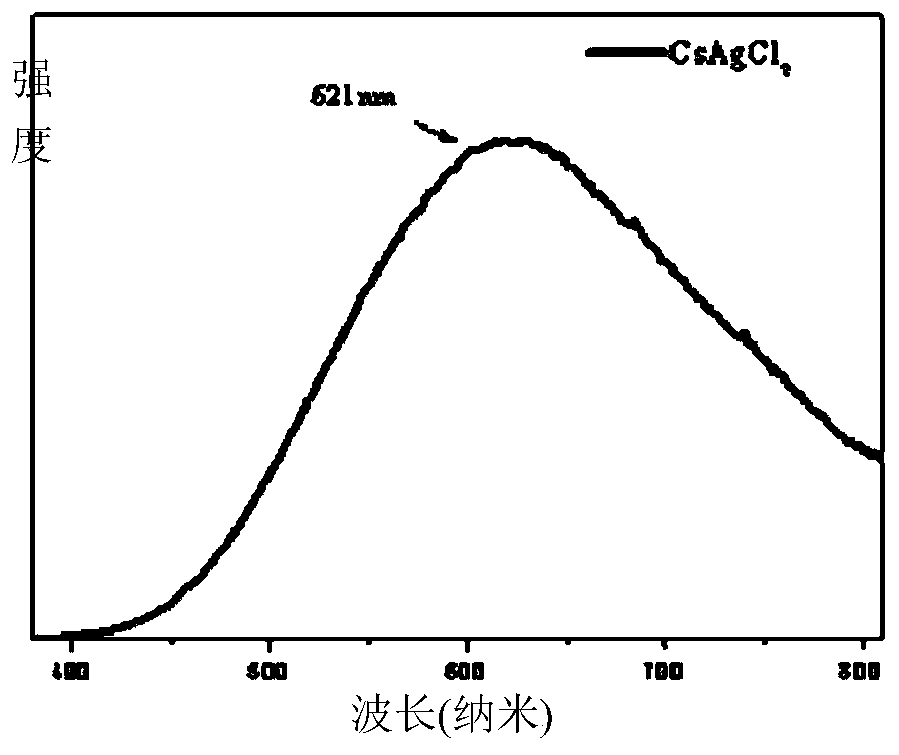
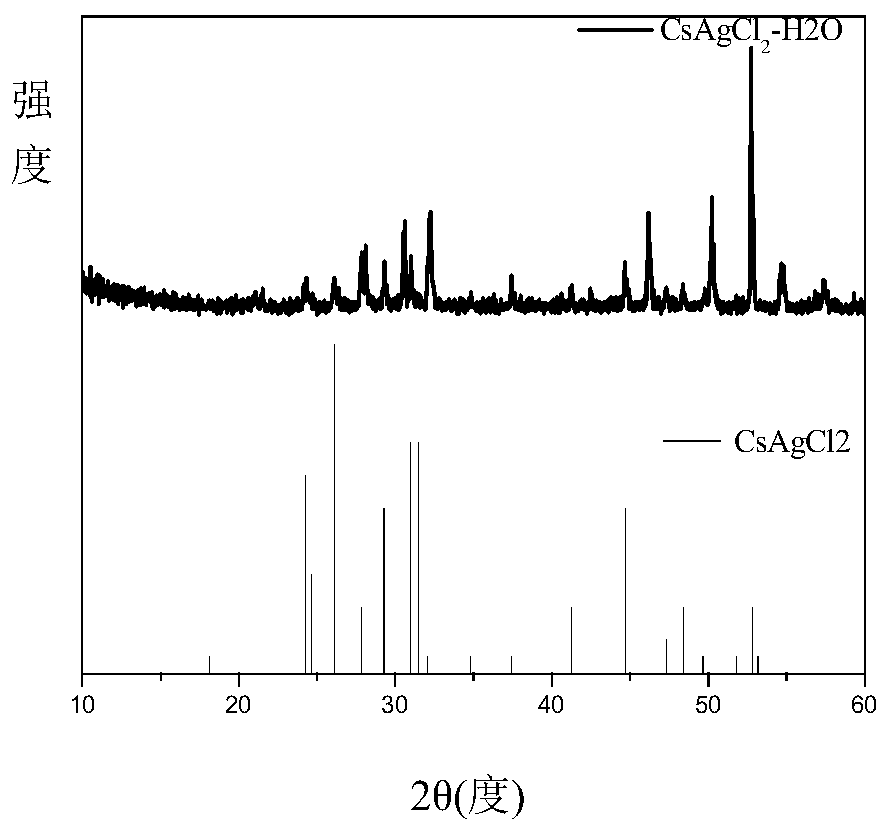
[0021] Put 1mmol cesium chloride, 1mmol silver chloride, 80ul oleylamine and 25 agate balls with a diameter of 6mm into a 25ml agate jar, set the AC frequency of the ball mill to 35Hz, and the speed at this time is 1050rad / min, and mechanically grind for 2h , the mixture gradually becomes dense from a fluffy white powder and adheres to the wall of the agate jar, then becomes soft again, and finally becomes a fluffy white powder again to obtain CsAgCl 2 The inorganic perovskite is irradiated with an ultraviolet lamp with an excitation wavelength of 254nm at this time, and it is found that the product emits yellow fluorescence. The obtained product was heat-treated in a vacuum oven at 240°C for 30 minutes, and cooled at -15°C for 1h to 2h, and the fluorescence intensity was obviously enhanced. The product was subjected to solid absorption analysis and fluorescence emission test, and its absorption spectrum was as follows: figure 1 Shown; the fluorescence emission spectrum is sh...
Embodiment 2
[0022] Embodiment 2: the amount of oleylamine in Example 1 is changed from 80ul to 40ul, 100ul, 120ul respectively, and other conditions and steps are unchanged, and the fluorescence efficiency of each product recorded is 53.8%, 59.5%, 56.7%, respectively, so The optimal amount of oleylamine is 80ul.
Embodiment 3
[0024] In Example 1, a drop of water was added to the solid powder mixture of cesium chloride and silver chloride before ball milling, and the ball milling time was shortened from 2 hours to 1 hour to obtain white fluffy CsAgCl 2 Inorganic perovskite powders, showing that for CsAgCl 2 In general, adding a small amount of water helps shorten the grinding reaction time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com