BFGF-loaded guided tissue regeneration membrane with core-shell structure and preparation method of bFGF-loaded guided tissue regeneration membrane
A technology that guides tissue regeneration and core-shell structure. It is applied in the field of biomedicine and can solve the problems of blended fibers that do not have drug control release, "burst release, and protein molecular structure destruction, etc., and achieve good cell compatibility and mild film-forming conditions. , the effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
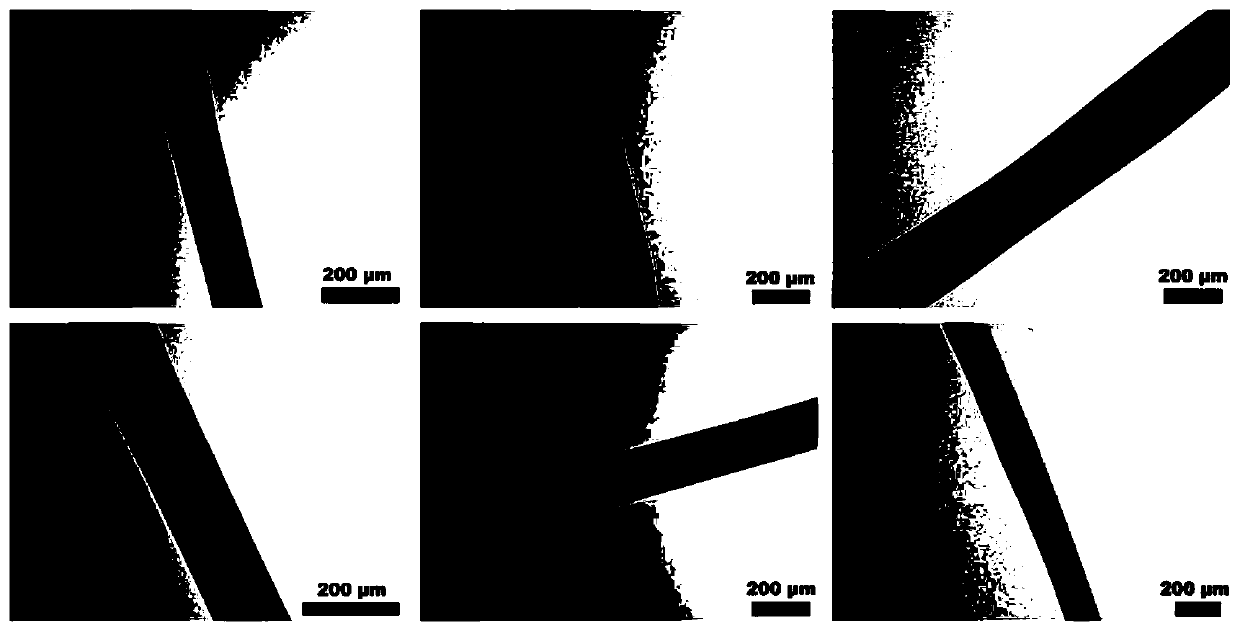
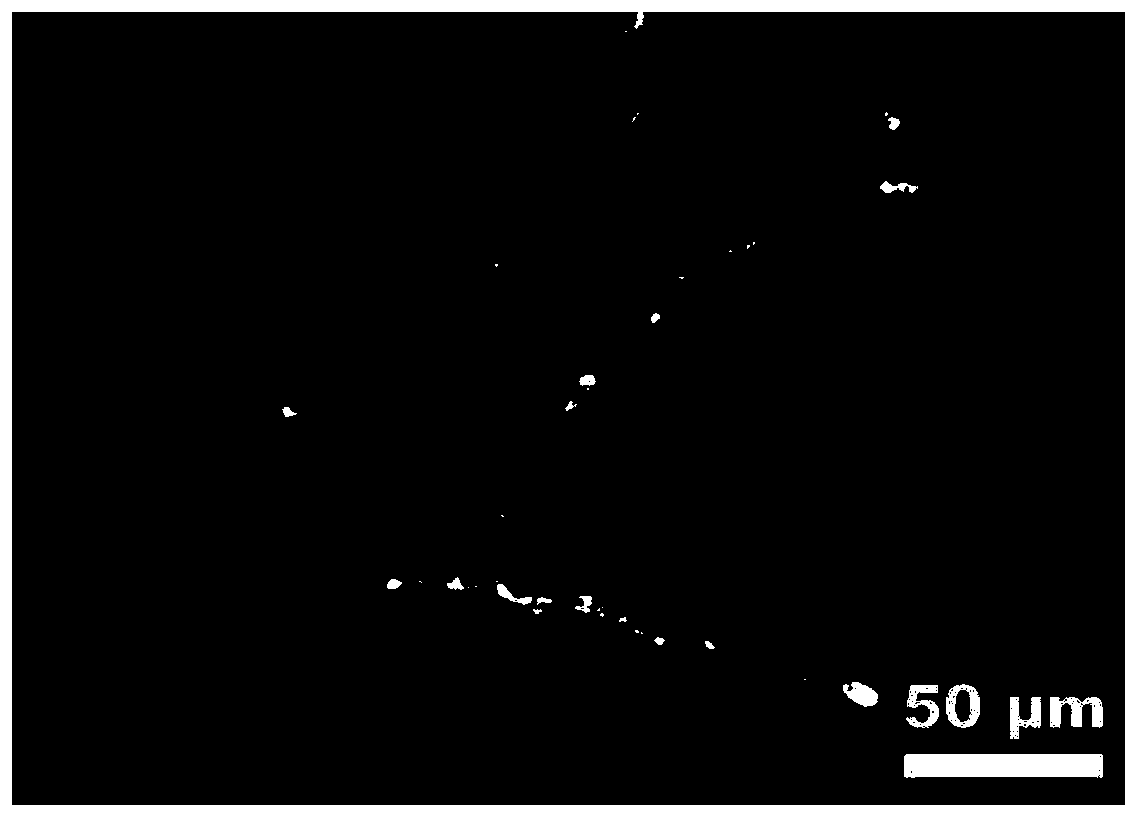
Image
Examples
Embodiment 1
[0078] 1) Configuration of aqueous solution: Dissolve basic fibroblast growth factor (bFGF) freeze-dried powder in phosphate buffered saline (PBS) containing bovine serum albumin (BSA) to form basic fibroblast growth factor / Bovine serum albumin solution, wherein the content of basic fibroblast growth factor (bFGF) is 0.5g / L, and the content of bovine serum albumin is 0.5% (w / v);
[0079] Dissolve dextran (DEX) in phosphate buffered saline (PBS), stir well to form a dextran solution with a concentration of 0.13g / mL;
[0080] Mix the basic fibroblast growth factor / bovine serum albumin solution with the dextran solution at a ratio of 1:15 to obtain a mixed solution of dextran / basic fibroblast growth factor / bovine serum albumin, which is used as the aqueous phase solution for subsequent use, and the final water The concentration of bFGF in the phase solution is 0.03g / L;
[0081] 2) Configuration of oil phase solution: Dissolve polylactic acid-glycolic acid copolymer (PLGA) in a ...
Embodiment 2
[0084] 1) Configuration of aqueous solution: Dissolve basic fibroblast growth factor (bFGF) freeze-dried powder in phosphate buffered saline (PBS) containing bovine serum albumin (BSA) to form basic fibroblast growth factor / Bovine serum albumin solution, wherein the content of basic fibroblast growth factor (bFGF) is 2.0g / L, and the content of bovine serum albumin is 0.5% (w / v);
[0085] Dissolve dextran (DEX) in phosphate buffered saline (PBS), stir well to form a dextran solution with a concentration of 0.12g / mL;
[0086] Mix the basic fibroblast growth factor / bovine serum albumin solution with the dextran solution at a ratio of 1:13 to obtain a mixed solution of dextran / basic fibroblast growth factor / bovine serum albumin, which is used as the aqueous phase solution for subsequent use, and the final water The concentration of bFGF in the phase solution is 0.15g / L;
[0087] 2) Configuration of oil phase solution: Dissolve polylactic acid-glycolic acid copolymer (PLGA) in a ...
Embodiment 3
[0090] 1) Configuration of aqueous solution: Dissolve basic fibroblast growth factor (bFGF) freeze-dried powder in phosphate buffered saline (PBS) containing bovine serum albumin (BSA) to form basic fibroblast growth factor / Bovine serum albumin solution, wherein the content of basic fibroblast growth factor (bFGF) is 4.0g / L, and the content of bovine serum albumin is 0.1% (w / v);
[0091] Dissolve dextran (DEX) in phosphate buffered saline (PBS), stir well to form a dextran solution with a concentration of 0.12g / mL;
[0092] Mix the basic fibroblast growth factor / bovine serum albumin solution with the dextran solution at a ratio of 1:14 to obtain a mixed solution of dextran / basic fibroblast growth factor / bovine serum albumin, which is used as the aqueous phase solution for subsequent use, and the final water The concentration of bFGF in the phase solution is 0.27g / L;
[0093] 2) Configuration of oil phase solution: Dissolve polylactic acid-glycolic acid copolymer (PLGA) in a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com