a kind of ab 2 Monomer and prepared donor-acceptor-donor-π bridge type hyperbranched conjugated polymer, preparation method and application thereof
A technology of hyperbranched conjugation and polymers, applied in semiconductor/solid-state device manufacturing, organic chemistry, electric solid-state devices, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
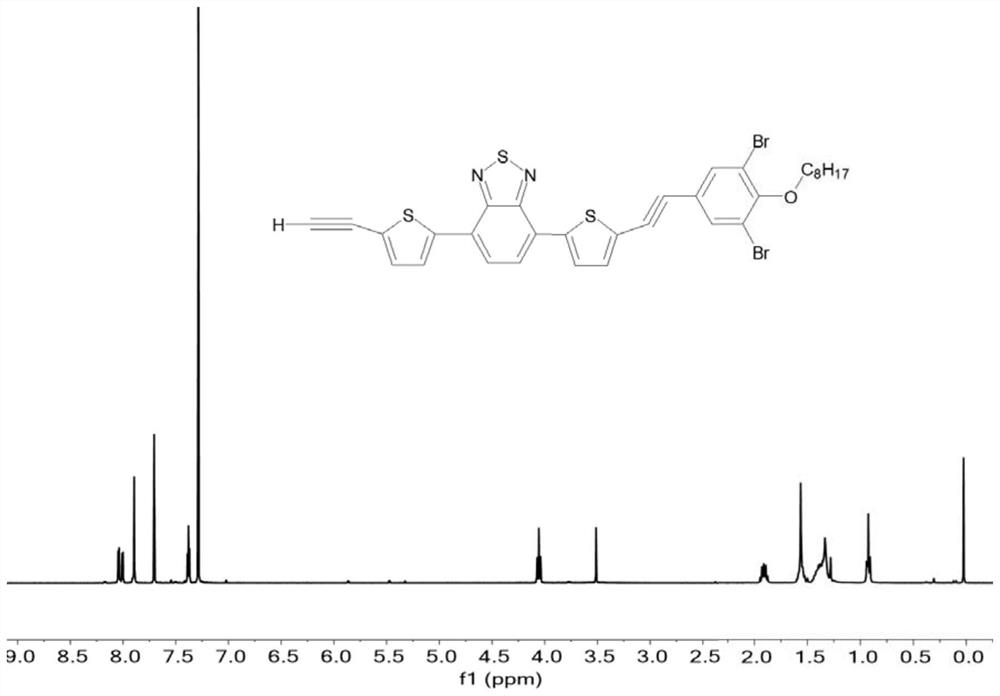
[0055] A kind of AB 2 Monomer, its structure is shown in formula I:
[0056]
[0057] where R is -C 8 h 17 , and its preparation process is as follows:
[0058]
[0059] The first step, the synthesis of 1,3-dibromo-5-ethynyl-2-octyloxybenzene (M1), the specific method reference (Journal of Organic Chemistry, 69(10), 3336-3339; 2004) and (Journal of PolymerScience, Part A: Polymer Chemistry, 56(1), 96-104; 2018).
[0060] In the second step, the D-A-D unit (4,7-bis(thiophen-2-yl)-2,1,3-benzothiadiazole) is replaced by iodine to obtain M2, the specific method reference (European Journal of Organic Chemistry, 2012(11), 2142-2151, S2142 / 1-S2142 / 93; 2012).
[0061] In the third step, M1 (9.00g), 228.8mg Pd (PPh 3 ) Cl 2 And 62.0mg CuI was added into a 500mL reaction flask, and vacuum pumping and nitrogen filling were repeated 3 times. Under the protection of nitrogen, 200 mL of THF that has been deoxidized and dehydrated and 1.80 mL of triethylamine were added success...
Embodiment 2
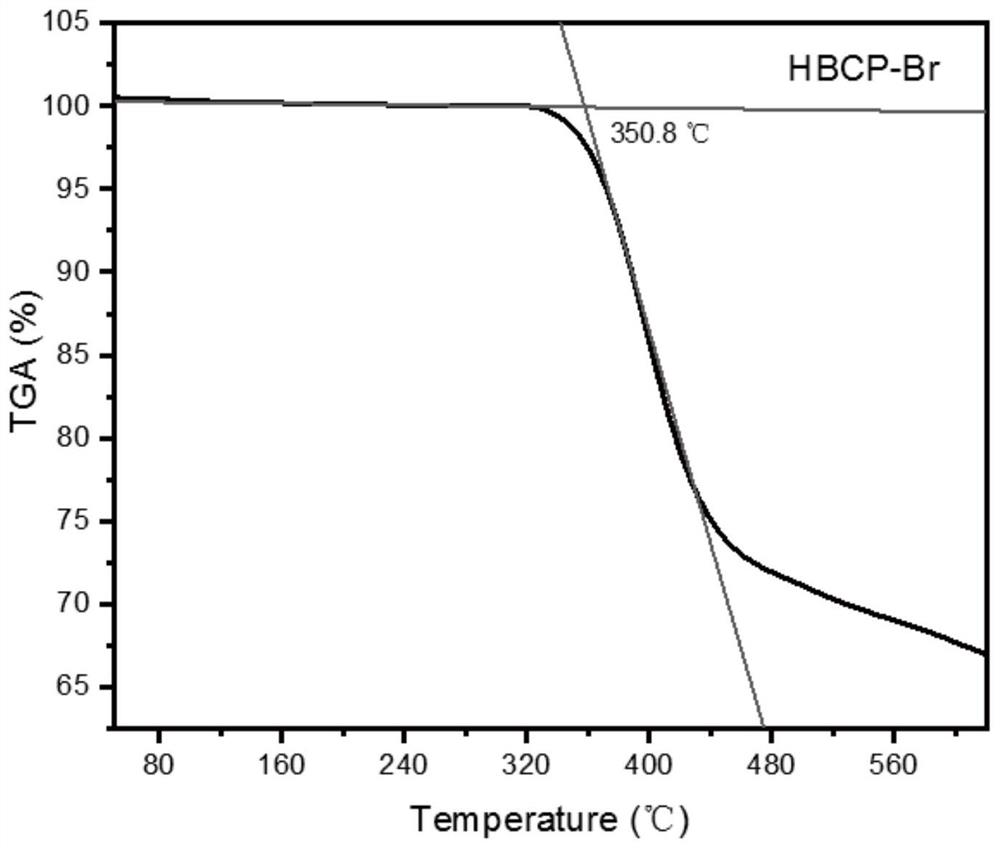
[0066] A kind of said donor-acceptor-donor-π bridge type (D-A-D-π type) hyperbranched conjugated polymer HBCP-Br, its structure is shown in formula II:
[0067]
[0068] where R is -C 8 h 17 .
[0069] Hyperbranched conjugated polymer HBCP-Br is based on the AB of embodiment 1 2 Monomer I is the raw material, prepared by Sonogashira coupling polymerization, the specific process is as follows:
[0070] Weigh AB 2 Monomer 1g, 30mg Pd(PPh 3 ) Cl 2 , 8mg CuI and 22mg PPh 3 Add it into the reaction bottle, repeat 3 times of vacuum pumping and nitrogen gas deoxygenation treatment, then add 20mL THF and 20mL triethylamine that have undergone strict dehydration and deoxygenation treatment, react under nitrogen protection at 60°C for 24 hours, filter, and remove the solvent under reduced pressure , the crude product was precipitated with petroleum ether and methanol successively, and the solid was collected and dried under vacuum to obtain 0.81 g of a hyperbranched polymer (H...
Embodiment 3
[0073] A kind of modification HBCP-CZ of described donor-acceptor-donor-π bridge type (D-A-D-π type) hyperbranched conjugated polymer, the hyperbranched polymer HBCP-Br prepared with embodiment 2 is The raw material is prepared by introducing a new donor or acceptor unit through the coupling reaction of its terminal -Br group. The specific process is as follows:
[0074] 100mg hyperbranched conjugated polymer HBCP-Br, 128mg 3-ethynyl-9-n-octylcarbazole, 8mg Pd(PPh 3 )Cl 2 , 2.14mg CuI and 4.43mg PPh 3 Add it into the reaction bottle, repeat the vacuum pumping and nitrogen gas removal treatment three times, then add 20mLTHF and 5mL triethylamine that have been strictly dewatered and deoxygenated, and react under nitrogen protection at 70°C for 24 hours. Filtrate with a short section of silica gel column, remove the solvent under reduced pressure, and then use petroleum ether and methanol to precipitate each time, collect the solid, and dry it under vacuum to obtain 91.2 mg of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com