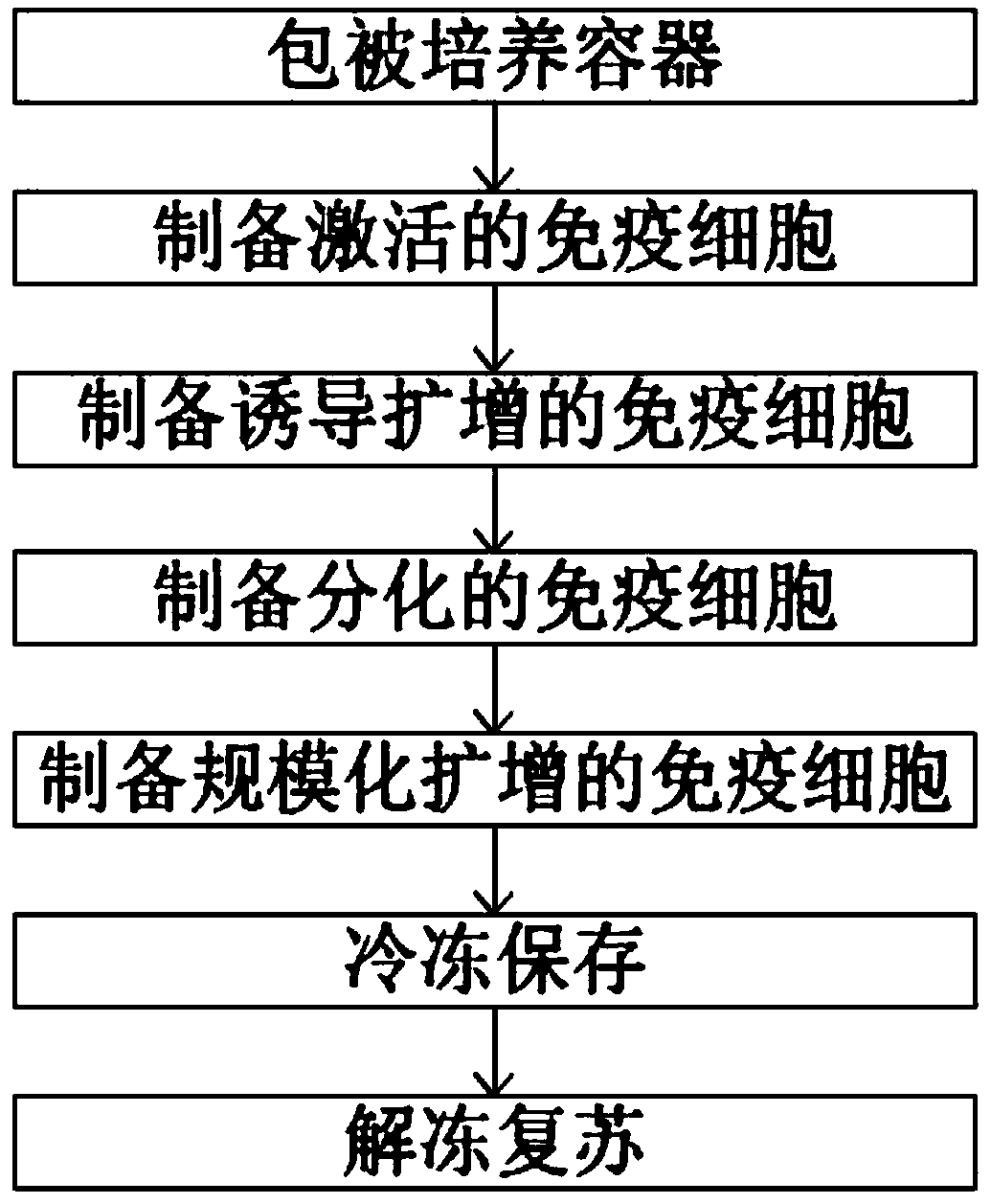
Immune cell in-vitro induction amplification method
A technology of immune cells and nuclear cells, which is applied in the field of cell culture, can solve the problems of low expansion multiple, poor immune effect, complicated culture system, etc., achieve high expansion multiple, good effect, and reduce safety risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A1~3
[0028] Embodiment A1~3: the configuration of immune cell activation medium
Embodiment A1
[0030] Use OpTmizer TMCTSTM serum-free medium as the basal medium, add relevant components to it, and make the components contained in the basal medium and their final concentrations: 8 volume percent autologous plasma, 500U / mL γ-interferon, 800U / mL interleukin-2 and 0.008KE / mL sapelin.
Embodiment A2
[0032] Use SuperCulture TmL500 human lymphocyte serum-free medium as the basal medium, add relevant components to it, and make the components contained in the basal medium and their final concentrations: 10 volume percent autologous plasma, 600U / mL γ-interference element, 1000U / mL interleukin-2 and 0.01KE / mL sapelin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com