Cell strain for efficiently expressing recombinant human erythropoietin and production process thereof
A high-efficiency expression and cell line technology, which is applied in the field of high-efficiency expression of recombinant human erythropoietin cell lines and production technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0121] Sample Preparation:
[0122] Take out the cryopreserved CHO cells from the liquid nitrogen tank. The CHO cells are purchased from ATCC. After resuscitation, they are inoculated into square flasks for cultivation. Stably grown cells were used as sample cells in this experimental example.
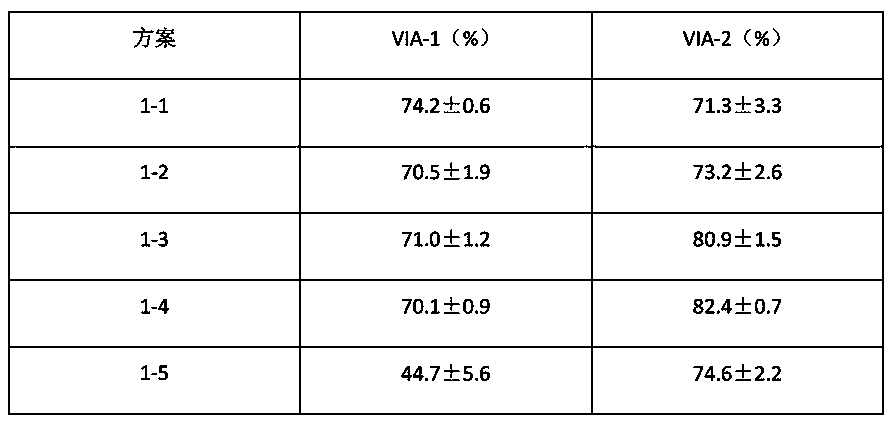
[0123] Scheme 1-1
[0124] S1: The sample cells were cultured in square flasks in F-12K medium containing 10% FBS, and the culture conditions were 37°C, 5% CO 2 Static culture; when the cell confluency reaches 70-80%, digest with trypsin for 30 seconds, add complete medium containing 15% FBS to stop digestion, collect the cells detached from the wall by centrifugation, and inoculate into a medium containing 5% FBS Square flask culture was carried out in F-12K medium at 37°C, 5% CO 2 Static cultivation;
[0125] S2: When the confluence of the cells reaches 70-80%, digest with trypsin for 30 seconds, add complete medium containing 15% FBS to stop the digestion, and collect the cells ...
experiment example 2
[0146] Sample Preparation:
[0147] The cryopreserved CHO cells were taken out from the liquid nitrogen tank, and the CHO cells were purchased from ATCC. After resuscitation, they were inoculated into F-12K medium containing 10% FBS for square bottle culture. The culture conditions were 37°C, 5% CO 2 Static culture; when the cell confluence reached 70-80%, the cells were collected and inoculated into F-12K medium containing 5% FBS for square flask culture. The culture conditions were 37°C, 5% CO 2 Static culture; when the cell confluence reached 70-80%, the cells were collected and inoculated into F-12K medium containing 1% FBS for square flask culture. The culture conditions were 37°C, 5% CO 2 Static culture; and the cells that grow stably in this medium are used as sample cells in this experimental example.
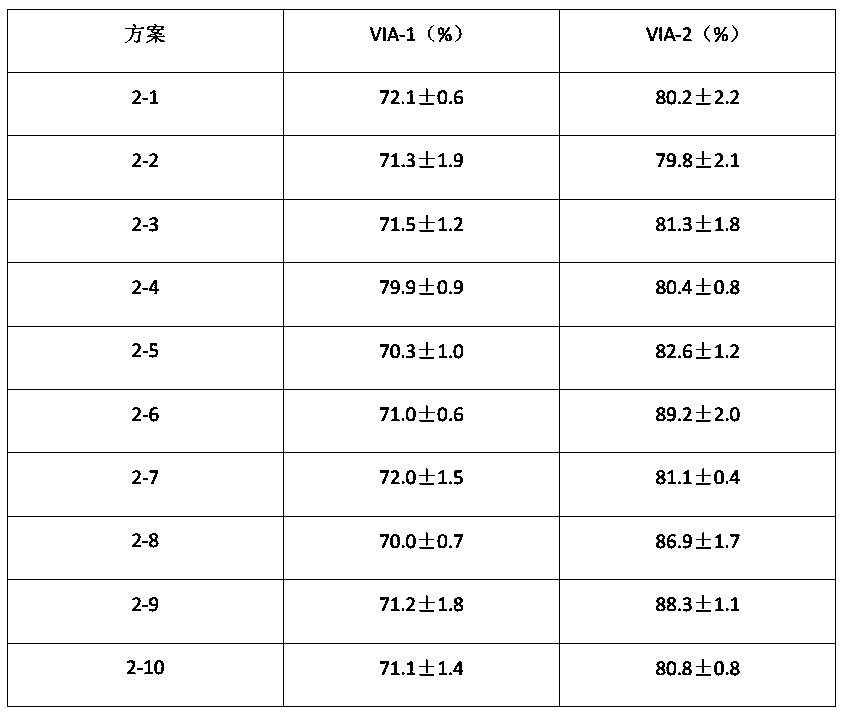
[0148] Scheme 2-1
[0149] S1: Culture the cells to be acclimated in a square flask in F-12K medium containing 1% FBS; when the confluence of the cells reaches 70-80%...
experiment example 3
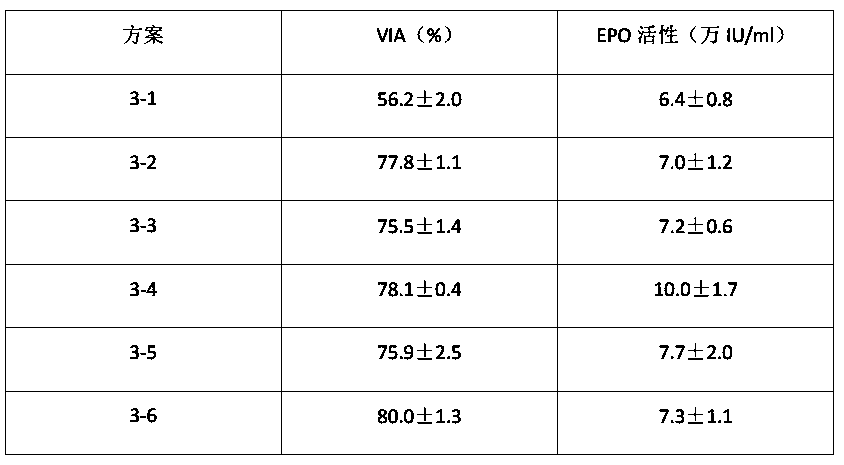
[0198] This experimental example tests the methods or steps in the third round of domestication.
[0199] Sample Preparation:
[0200] D1: Take out the frozen CHO cells from the liquid nitrogen tank. The CHO cells are purchased from ATCC. After resuscitation, they are inoculated into F-12K medium containing 10% FBS for square bottle culture. The culture conditions are 37°C, 5% CO 2 Static culture; when the cell confluence reached 70-80%, the cells were collected and inoculated into F-12K medium containing 5% FBS for square flask culture. The culture conditions were 37°C, 5% CO 2 Static culture; when the cell confluence reached 70-80%, the cells were collected and inoculated into F-12K medium containing 1% FBS for square flask culture. The culture conditions were 37°C, 5% CO 2 Static culture; when the cell confluency reaches 70-80%, digest with trypsin for 30 seconds, add complete medium containing 15% FBS to stop the digestion, centrifuge to collect the cells detached from t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com