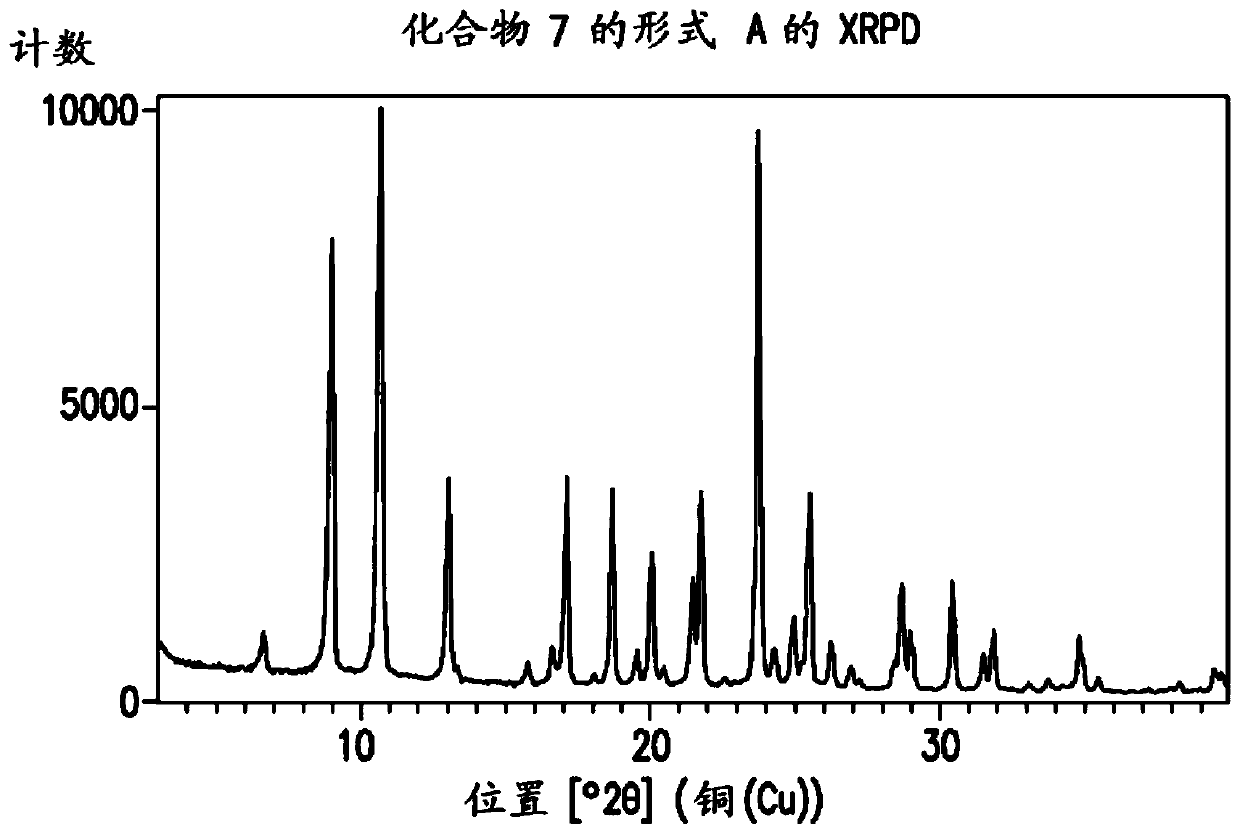
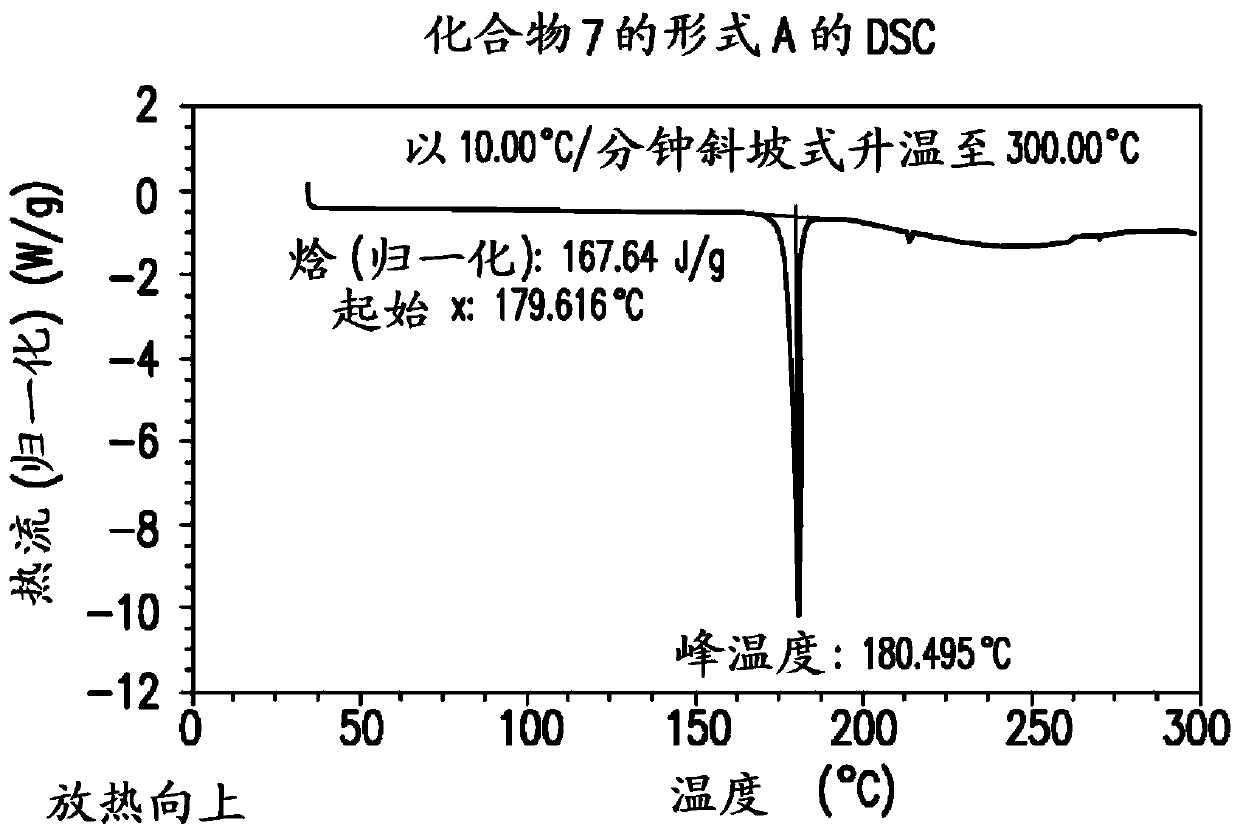
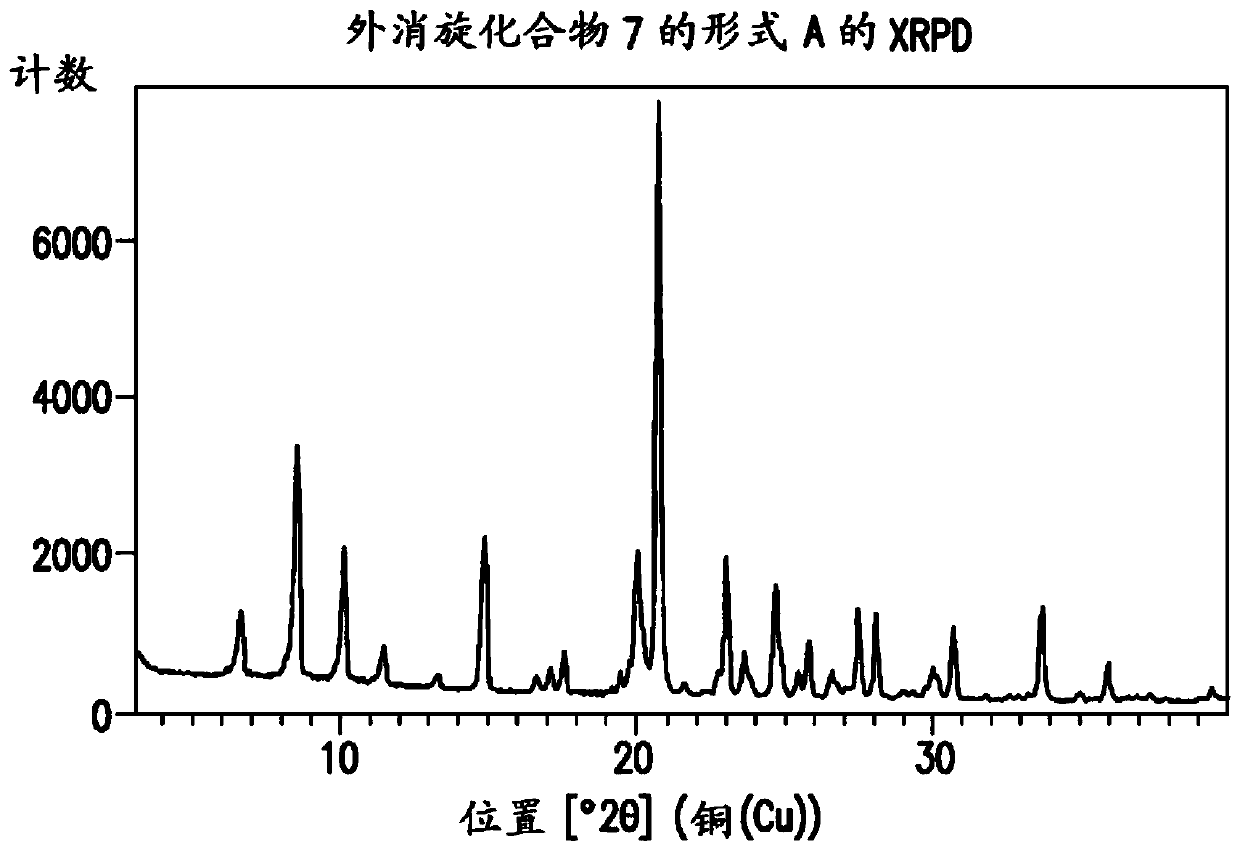
Processes for preparation of (s)-tert-butyl 4,5-diamino-5-oxopentanoate
A technology, the technology of amino protecting group, is applied in the technical field of preparing (S)-4,5-diamino-5-oxopentanoic acid tert-butyl ester, which can solve the problem that the chiral purity analysis data is not well understood issues such as definition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0636]
[0637] In one embodiment, there is also provided herein a process for the preparation of a compound of formula (XV) or a salt, solvate, hydrate, enantiomer, mixture of enantiomers or isotopologues thereof comprising 2-chloro-6-hydroxyl Carboxymetallation of benzaldehyde (eg, Pd / CO / MeOH). One embodiment of this method is described in the following scheme:
[0638]
[0639] or related variants (using amines instead of alcohols ROH to form amides)
[0640] 5.2.8 Preparation of O-t-Bu-DIC isourea
[0641] In one embodiment, provided herein is a process for the preparation of O-t-Bu-DIC isourea of the following structure:
[0642]
[0643] The process involves reacting diisopropylcarbodiimide (DIC) with t-butanol and Cu(I) salt in the presence of oxygen.
[0644] In one embodiment, oxygen is present in an amount up to about 22% of the atmosphere. In one embodiment, oxygen is present in an amount from about 1% to about 10% of the atmosphere. In one embodimen...
Embodiment 1
[0746]
[0747] Step 1: Synthesis of compound 3
[0748] Add water 60ml to L-glutamic acid (2, 10g, 68.0mmol). To the resulting suspension was slowly added benzyl chloroformate (CbzCl, 9.16ml, 63.9mmol). Simultaneously 2N NaOH (60 mL) was added to maintain the pH at 10-12. The rate of addition was adjusted to maintain a batch temperature of 0-5 °C. After addition of CbzCl, the mixture was stirred at 5°C for 1 hour and then at 20 to 25°C for a further 16 hours. The mixture was then washed with EtOAc. The aqueous product-containing layer was acidified to pH = 2-3 by addition of cone. HCl, then extracted into EtOAc. The organic layer was washed with Na 2 SO 4 Dry and concentrate under reduced pressure. The resulting solid was dried overnight in a 35-40 °C oven to yield 16 g of compound 3 (84%). LC-MS: Calcd. m / e(M+1), 282.1; Found: 282.2. 1 H NMR (DMSO-d 6 ,): δ (ppm) = 1.68-1.83 (m, 1H), 1.91-2.03 (m, 1H), 2.27-2.35 (m, 2H), 3.99-4.22 (m, 1H), 5.03 (s, 2H) , 7.29-7...
Embodiment 2
[0759]
[0760] Example 2 differs from Example 1 in the choice of amine nucleophile used to open the lactone. In Example 2 ammonia was used directly, whereas in Example 1 a protected amine was used.
[0761] Step 3: Synthesis of compound 7
[0762] To a solution of compound 4 (1.1 g, 3.75 mmol) in methanol (20 ml) was added ammonium hydroxide (20-30 wt%, 10 ml, 20 equiv) at 20 to 25°C. The mixture was stirred at 20-25°C for 5 days. The mixture was concentrated under reduced pressure to remove methanol. The resulting oil was dissolved in water. The mixture was washed with EtOAc, then the aqueous layer was acidified with 35% HCl to pH = 2-3. The acidified mixture was extracted with EtOAc (20ml x 3). The organic layer was washed with MgSO 4 Dry and concentrate on a rotary evaporator. The resulting solid was dried in an oven under vacuum to afford compound 7. LC-MS m / e calcd. 281.1 (M+1); found 281.2. 1 H NMR (DMSO-d 6 ,): δ (ppm) 1.65-1.78 (m, 1H), 1.82-1.95 (m, 1H),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com