Safe coxsackie virus for treating KRAS mutant tumors, and pharmaceutical composition thereof
A technology of coxsackie virus and composition, which is applied in the field of medicine and can solve the problem that therapeutic agents cannot be safely administered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Coxsackie virus preparation
[0103] 1.1 Sequence synthesis and plasmid construction:
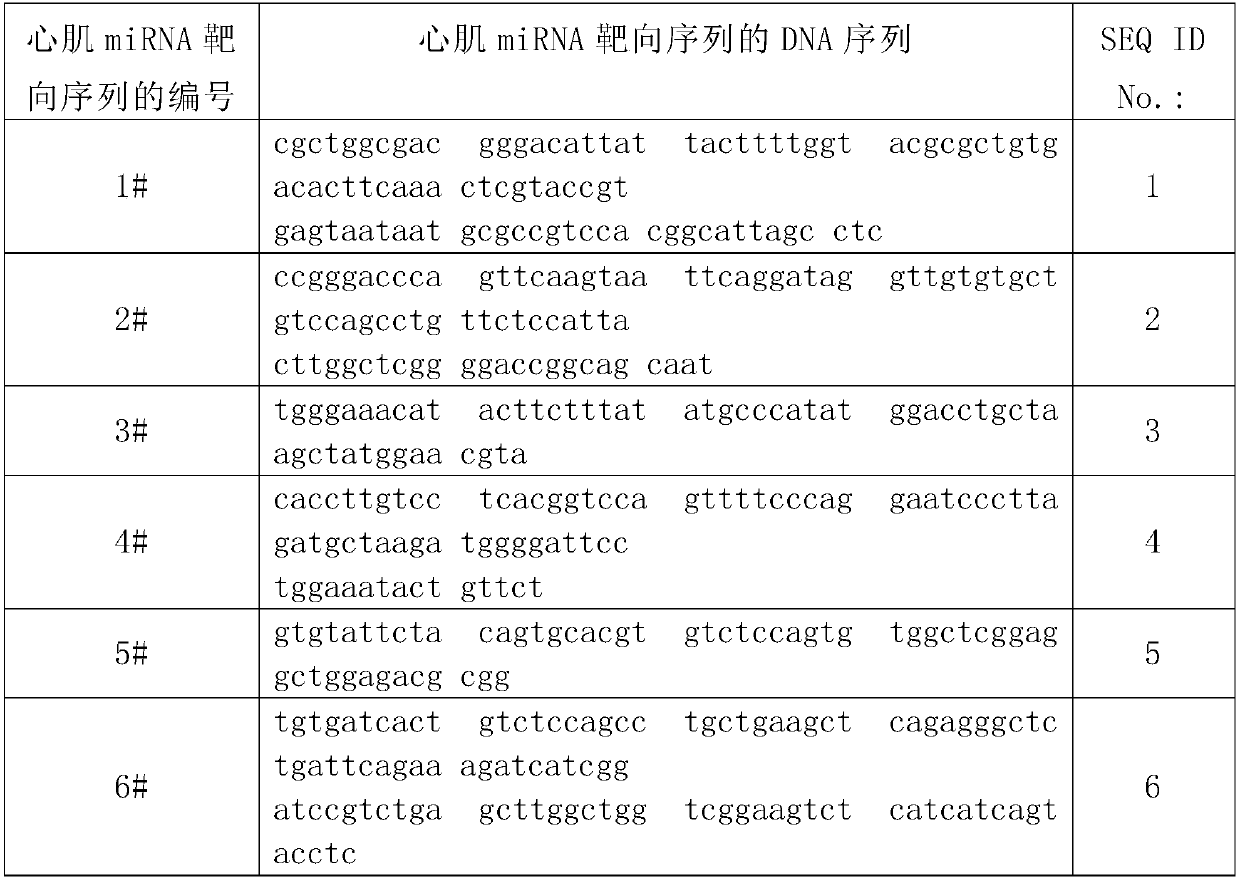
[0104] The DNA sequence corresponding to the myocardial miRNA targeting sequence shown in Table 1 was synthesized by artificial synthesis method:
[0105] Table 1 DNA sequence of myocardial miRNA targeting sequence
[0106]
[0107] Containing Sfi I site, artificial 3C pro / 3CD pro Cleavage site and pMKS1 plasmid encoding full-length cDNA of CVB3 (Wodrolf strain) (available from J. Lindsay Whitton (The Scripps Research Institute, LaJolla, California)). The SfiI site in this plasmid serves as an accepting cloning site for insertion of the appropriate oligonucleotide sequence.
[0108] Complementary sequences containing three miRNAs (respectively denoted as miR-A, miR-B and miR-C) (i.e. myocardial miRNA targeting sequence) were generated by PCR, which were continuously incorporated into the 3'UTR of pMKS1 to generate plasmid pMKS1 - A-B-C (CVB3-miRNA). The resulting plasmid wi...
Embodiment 2
[0130] Symptoms of the virus
[0131] 2.1 Virus growth characteristics
[0132] For the one-step growth curve of the virus, it is determined as follows:
[0133] a) after inoculating HeLa cells to form a monolayer, infect HeLa cells with the virus CVB3 obtained in Example 1;
[0134] b) Place virus-infected cells at 37°C 5% CO 2 In the incubator, at different time points, the cell supernatant was collected to detect the virus titer;
[0135] c) Draw a change curve of the virus gradient with the culture time, that is, a one-step growth curve.
[0136] CVB3 is a cytolytic virus. The earlier its progeny virus appears in the cell culture supernatant, the faster the titer rises. The higher the titer, the lower the survival rate of sensitive cells, and the corresponding coxsackie virus mutant strain is cytolytic. The higher the ability.
[0137] 2.2 Cytotoxicity test
[0138] Different kinds of tumor cells were inoculated in 12-well plates. When the cell density reached 90%, r...
Embodiment 3
[0150] Performance testing of Coxsackie virus
[0151] 3.1 Western blotting:
[0152] In order to further evaluate whether the Coxsackievirus of the present invention can inhibit the synthesis of viral proteins in normal cell lines without restricting the viral replication in PC cells, the viral protein 1 (VP1) and cleaved caspase-3 (cells) were determined by Western blot analysis. A marker of death) protein expression levels. CVB3-miRNA infected cells will be harvested and lysed. Briefly, equal amounts of proteins will be subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to nitrocellulose membranes. The resulting membranes will be incubated with primary antibodies to VP1, cleaved Caspase-3 and β-actin. Protein levels of VP1 and cleaved caspase-3 will be quantified by densitometric analysis using NIH ImageJ and then normalized to β-actin.
[0153] The results show that the Coxsackie virus of the present invention hardly ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com