Efficacy of a gastro-retentive bile acid sequestrant dosage form
A technology of bile acid chelating agent and gastric retention, which is applied in the direction of drug combination, pill delivery, medical preparations containing active ingredients, etc., and can solve the problem of incomplete response to PPI treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0184] Example 1: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Dose-Ranging Finding Trial to Determine Oral Administration of Bile Acid Sequester Formulations in Patients with Symptomatic GERD Incompletely Responsive to Proton Pump Inhibitors for 8 Weeks safety and efficacy.
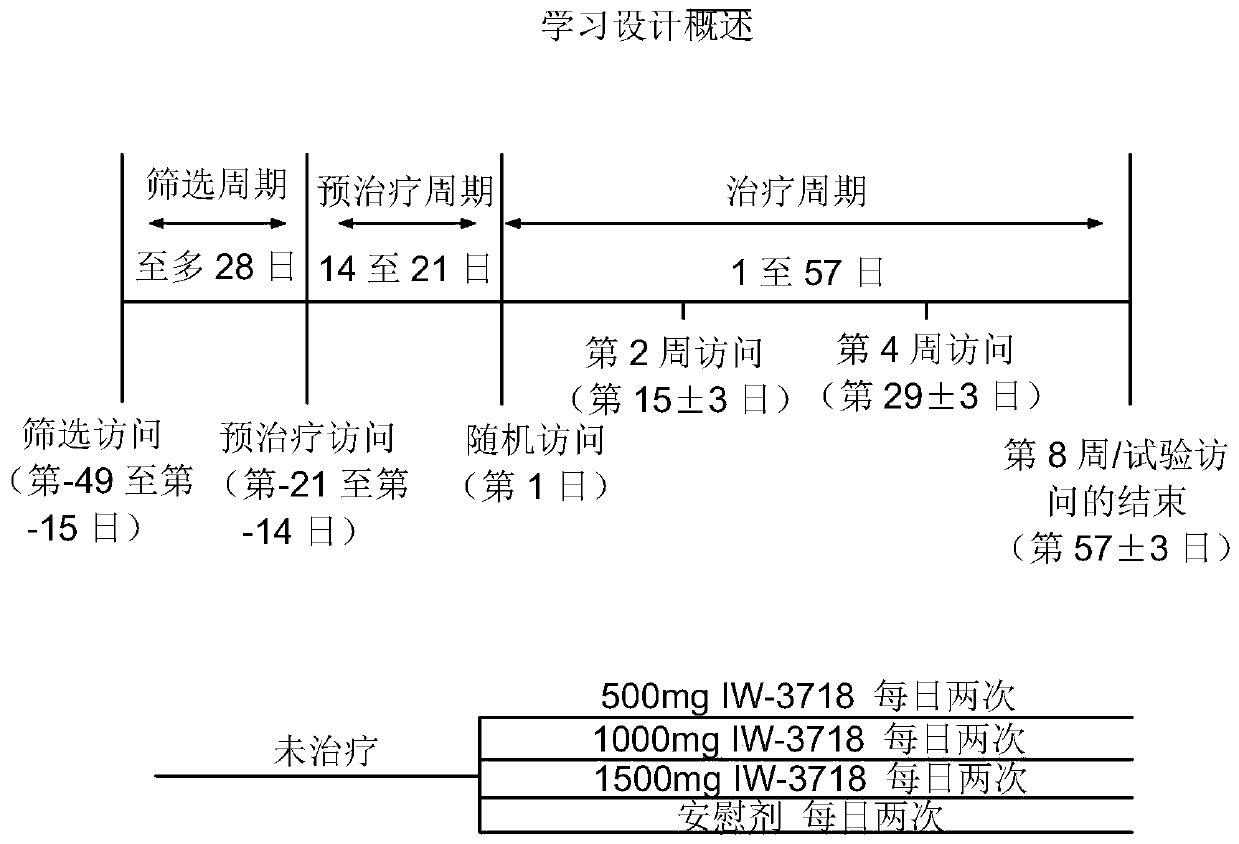
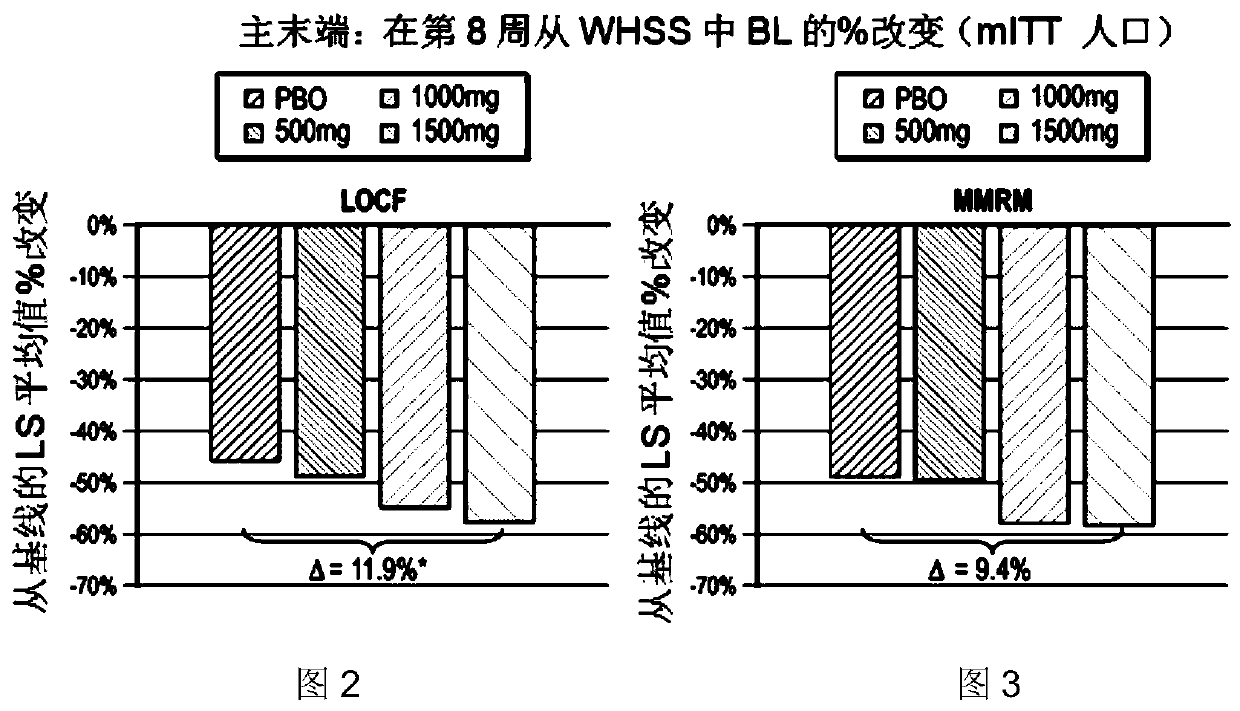
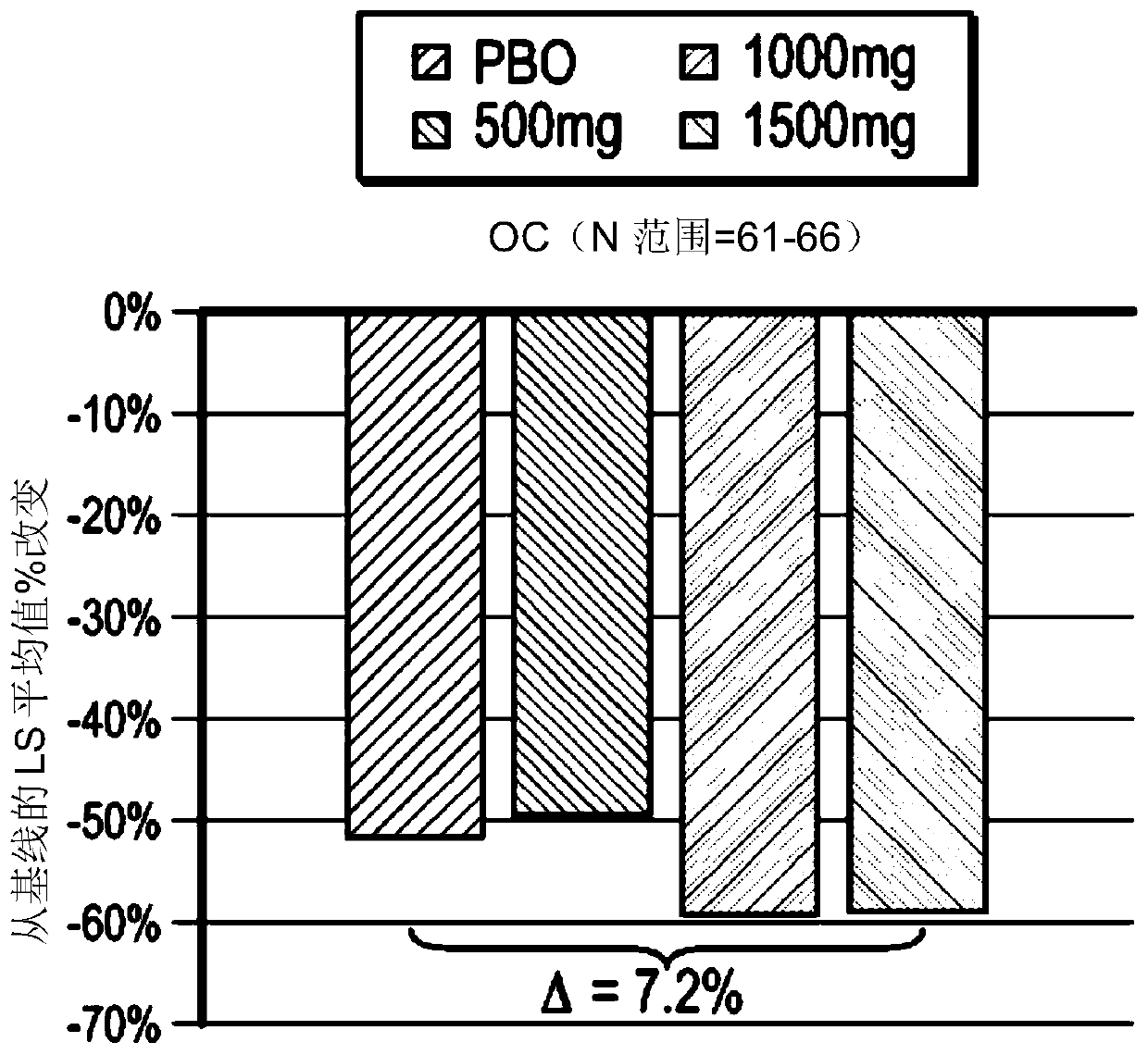
[0185] This example presents the protocol and results of a multicenter (approximately 60-80 centers in the United States), randomized, double-blind, placebo-controlled, parallel group, 8-week study consisting of 3 distinct periods (screening period up to 28 days; pretreatment period 14-21 days; treatment period 57 days). Such as figure 1 , the study included patients who had GERD and continued to experience GERD symptoms while receiving once-daily treatment with a proton pump inhibitor at the standard dose that the investigators considered optimized. Eligible patients continued to take PPIs and were randomized to either placebo or IW-3718 500 mg twice daily, IW-3718 1000 mg twice dai...
Embodiment 2
[0793] Example 2: A single-center, open-label, randomized, single-dose, 3-channel scintigraphy study in healthy subjects with 3 periods, each with a different phase designed to evaluate the in vivo performance of IW-3718 The in vivo performance of the breakfast composition, IW-3718, will be compared to that of an immediate release bile acid sequestrant in the fed state.
[0794] The purpose of this study was to compare the gastroprotective properties of a 500 mg IW-3718 tablet in the fed state compared to an immediate release formulation (Comparative Product Immediate Release Cholestagel [bile acid sequestrant; 625 mg]) . The gastrointestinal maintenance properties of the two drugs were studied after breakfast with different fat and calorie contents.
[0795] The recommended cholesterol dose is 6 x 625 mg tablets per day; the dose chosen in this study was 1 x 625 mg tablet in each of the 3 periods. This dose was well within the recommended daily dose, limiting exposure to he...
Embodiment 3
[1104] Example 3, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Dose-Range Exploratory Trial to Determine the Safety and Efficacy of Oral Bile Acid Sequestration Formulations in Patients with Gastroesophageal Reflux Disease for Up to 8 Weeks
[1105] eligibility criteria
[1106] Inclusion criteria
[1107] Each patient must meet all of the following criteria to be eligible to participate in this study:
[1108] Patients had signed the ICF prior to performing any study-specific procedures.
[1109] Patients were nonhospitalized male or female (if female, not pregnant) and at least 18 years old at the screening visit.
[1110] Patients had a diagnosis of GERD and reported experiencing GERD symptoms (heartburn or regurgitation) on average > 4 days per week in the last 8 weeks prior to the Screening Visit.
[1111] Patients had received standard labeled dose, QD, PPI therapy (which, in the investigator's judgment, could not be further improved by changing the b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com