A kind of preparation method of canagliflozin hemihydrate
A hydrate and total water technology, applied in organic chemistry methods, organic chemistry and other directions, can solve the problems of low water content upper limit canagliflozin monohydrate and other problems, and achieve the effect of solving product quality instability and reducing production process requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Preparation of canagliflozin anhydrate
[0035] Weigh 72g of canagliflozin hemihydrate, add 1000mL of ethyl acetate and 1000mL of purified water, and stir at room temperature or properly heated at 35-40°C until completely dissolved. The layers were allowed to stand, the aqueous layer was discarded, and the organic layer was evaporated to remove the solvent. After dissolving with ethyl acetate, the solvent was evaporated by rotary evaporation at 35-40° C., and the residual water was taken away repeatedly several times. Finally, under the vacuum of the oil pump, the remaining solvent was evaporated to dryness to obtain the anhydrous compound of canagliflozin in the form of white foam.
Embodiment 2
[0037] Add 7.06g (15.88mmol) of canagliflozin anhydrous compound obtained in Example 1 and 0.46ml (7.94mmol, 0.5eq) of glacial acetic acid to 90mL of ethyl acetate previously dried with molecular sieves, and seal with a rubber stopper. When the solid is completely dissolved, its Karl's moisture is measured to be 0.02%. Add 1.13ml of water (that is, the water content is 4 molar equivalents), and stir evenly. Add 75 mL of n-heptane dropwise at room temperature, and crystallize for one hour after dropping, then lower the temperature, filter under reduced pressure, and heat the obtained solid under vacuum for two hours to obtain the final product. Its Karl's moisture is measured to be 2.03%, and it can be determined that the obtained solid is canagliflozin hemihydrate.
Embodiment 3
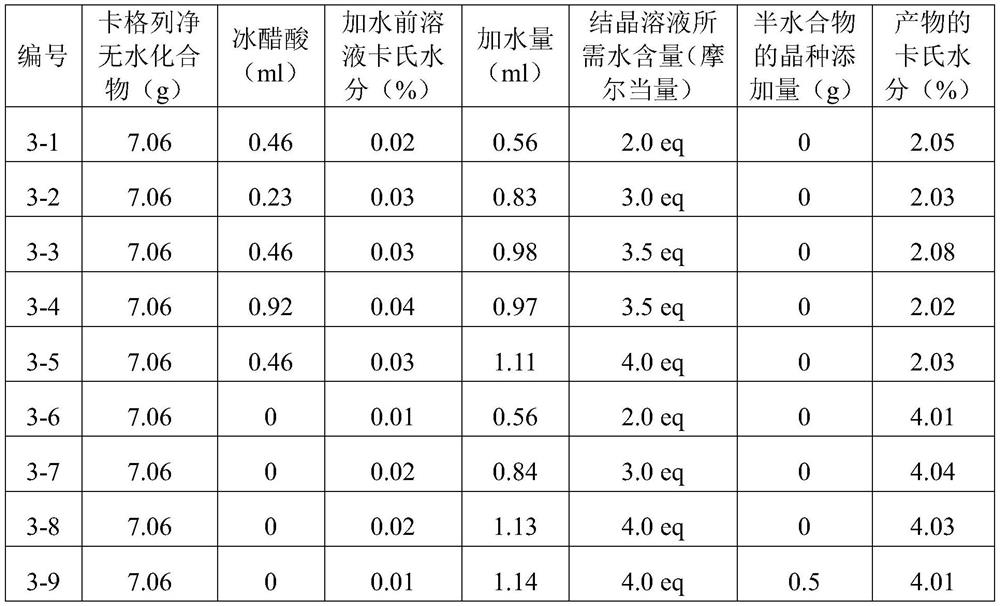
[0039] Referring to the method of Example 2, change the amount of glacial acetic acid and additional water, and detect the Karl's moisture in the final product. The specific data are shown in Table 1.
[0040] Table 1
[0041]
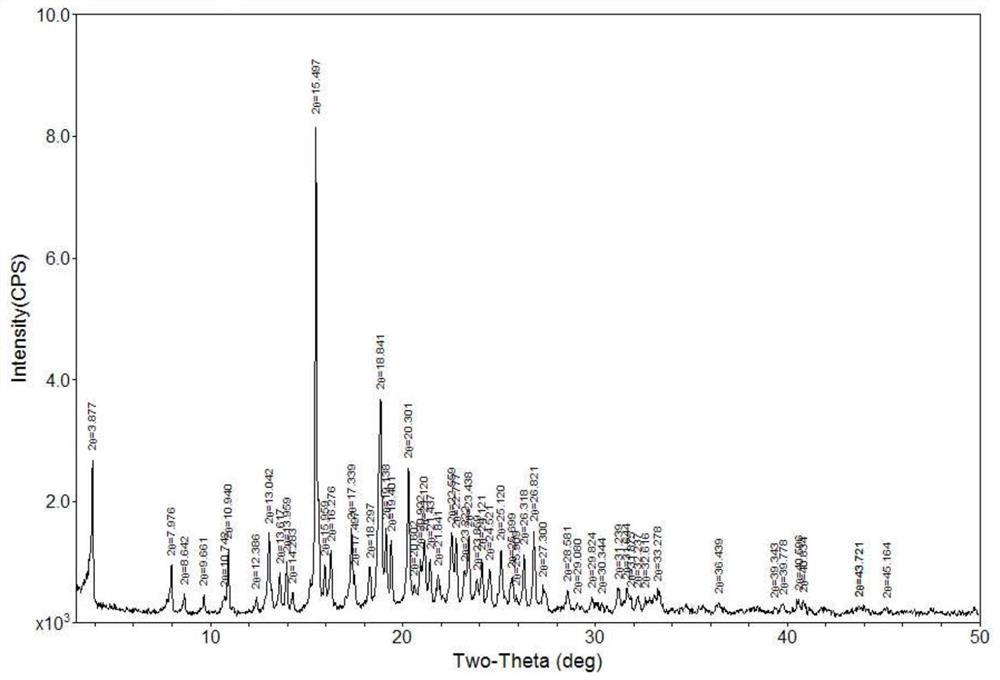
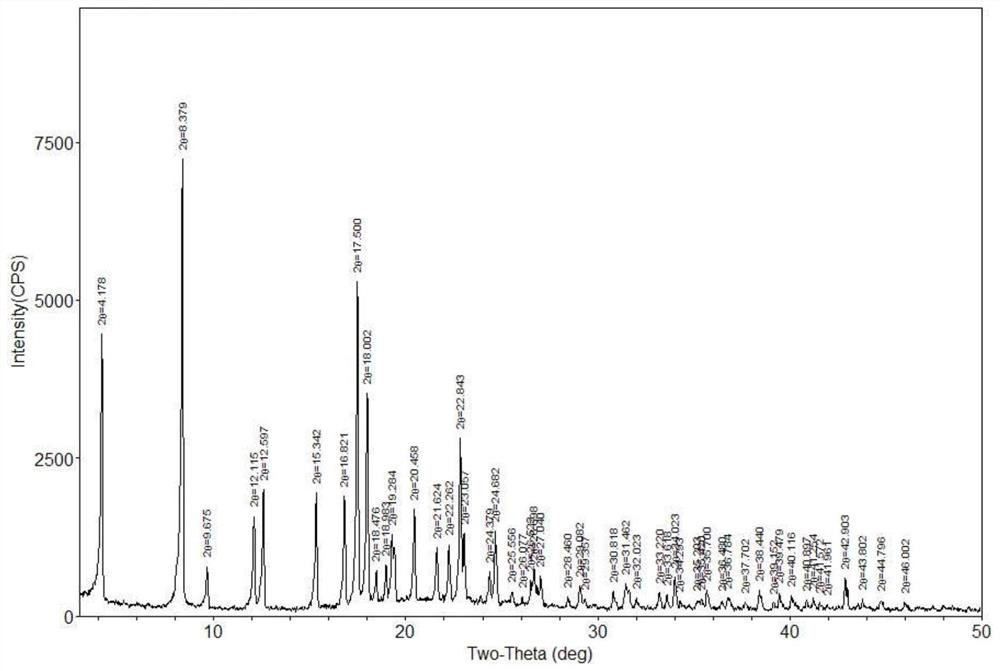
[0042] The Karl's moisture of completely dried canagliflozin hemihydrate is 1.98%, and the Karl's moisture of completely dried canagliflozin monohydrate is 3.89%. Therefore, it can be judged that the products obtained in 3-1 to 3-5 are all canagliflozin hemihydrates, and the products obtained in 3-6 to 3-8 are canagliflozin monohydrates by analyzing the Karl’s water content of the products. In order to further confirm its crystal form, the PXRD of each product was also measured, and the results were consistent with the results of Karl Fischer's moisture determination. Wherein the PXRD of the products of 3-5 and 3-8 are respectively as follows figure 1 , figure 2 shown.
[0043]The results show that when the crystallization solution contains ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com