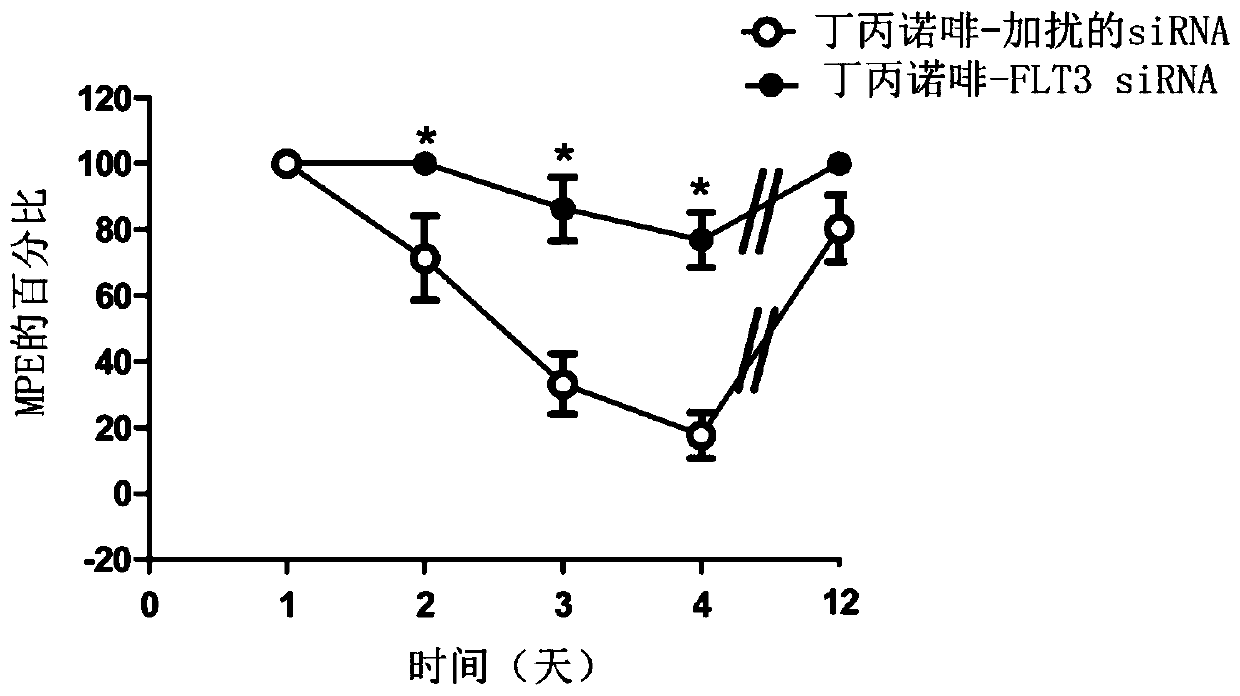
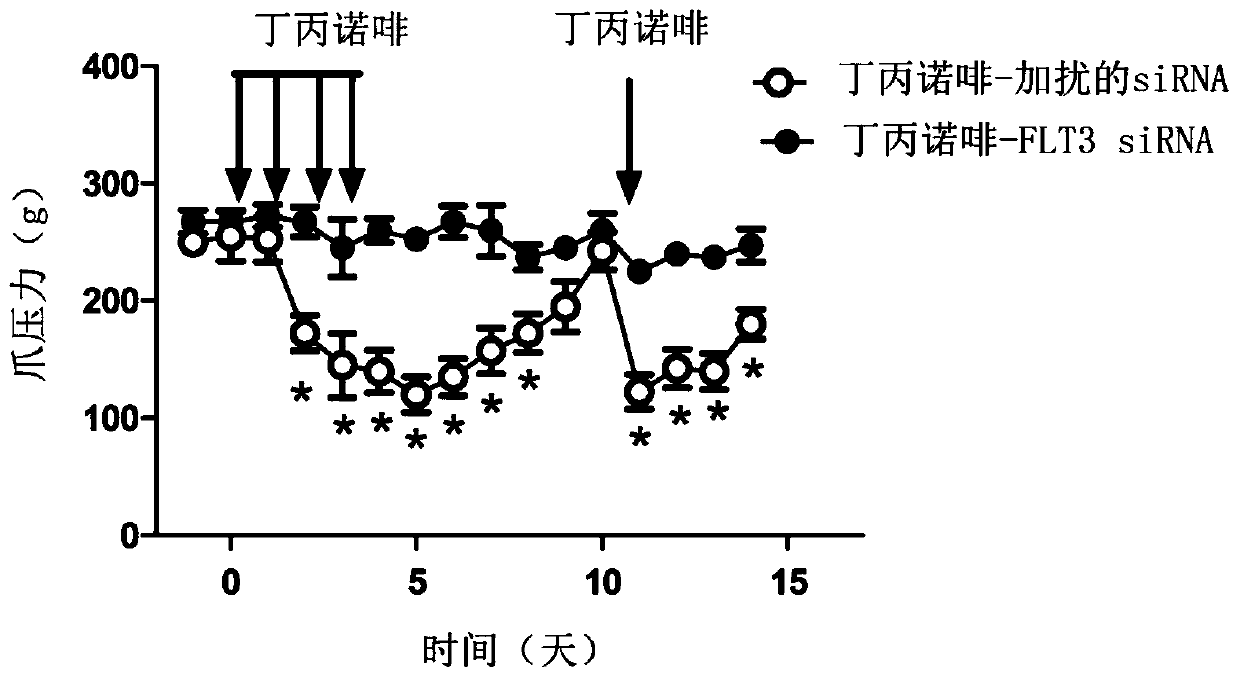
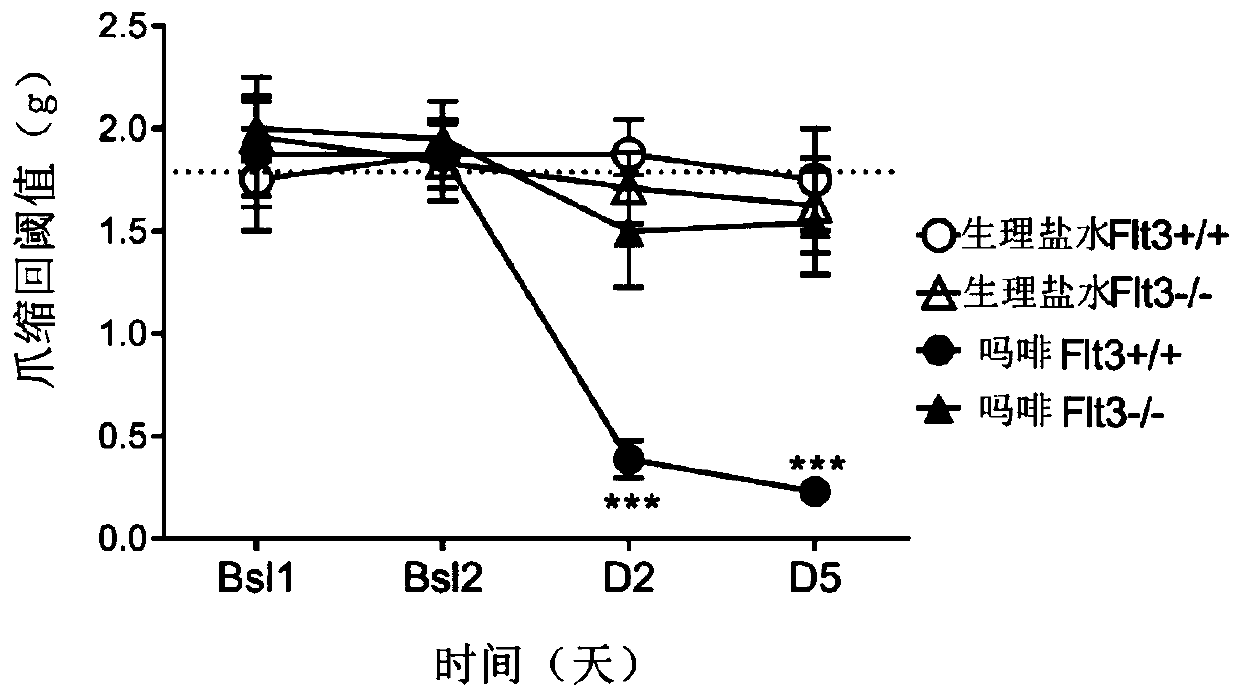
FLT3 inhibitors for improving pain treatments by opioids
A technology of opioid drugs and inhibitors, applied in the field of compounds and compositions with efficacy and prevention and treatment of their side effects, which can solve the problems of patients not receiving pain treatment, quality of life, increasing opioids, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 4 approach
[0017] According to a fourth embodiment, the invention relates to a pharmaceutical combination comprising a FLT3 inhibitor and an opioid.
[0018] According to a fifth embodiment, the present invention relates to a pharmaceutical combination comprising a FLT3 inhibitor, in particular a compound of formula (I) or (II) as described below, more particularly a FLT3 inhibitor compound specifically listed below, even More particularly N-(5-chloro-2-hydroxyphenyl)-3-(piperidin-1-ylsulfonyl)benzamide and for separate administration, over time, or Concomitantly administered opioids.
no. 6 approach
[0019] According to a sixth embodiment, the present invention relates to a pharmaceutical kit especially intended for the treatment of pain, comprising:
[0020] (i) a first galenic formulation comprising a FLT3 inhibitor, in particular a compound of formula (I) or (II) as described below, more particularly a FLT3 inhibitor compound specifically listed below, even more particularly is N-(5-chloro-2-hydroxyphenyl)-3-(piperidin-1-ylsulfonyl)benzamide, and
[0021] (ii) A second galenic formulation comprising an opioid.
[0022] Compounds that improve the efficacy and safety of opioids
[0023] As used herein, the terms "FLT3" or "FLT3 receptor" (Fms-related tyrosine kinase 3), also known as CD135, Ly72, Flk-2, Flt-3 or B230315G04, are used interchangeably and have Its ordinary meaning in this field. The FLT3 receptor may be from any animal species, but is typically a mammalian (eg human and non-human primate) FLT3 receptor, especially a human FLT3 receptor. The naturally o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com