Moist heat sterilization method of citicoline and sodium salt injection thereof
A technology of moist heat sterilization and citicoline, which is applied in heating, disinfection, etc., can solve the problems of drug safety risks, accelerate the degradation of main components of drugs, and increase the growth of impurities, so as to shorten the sterilization time, improve safety, and ensure sterilization. The effect of bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
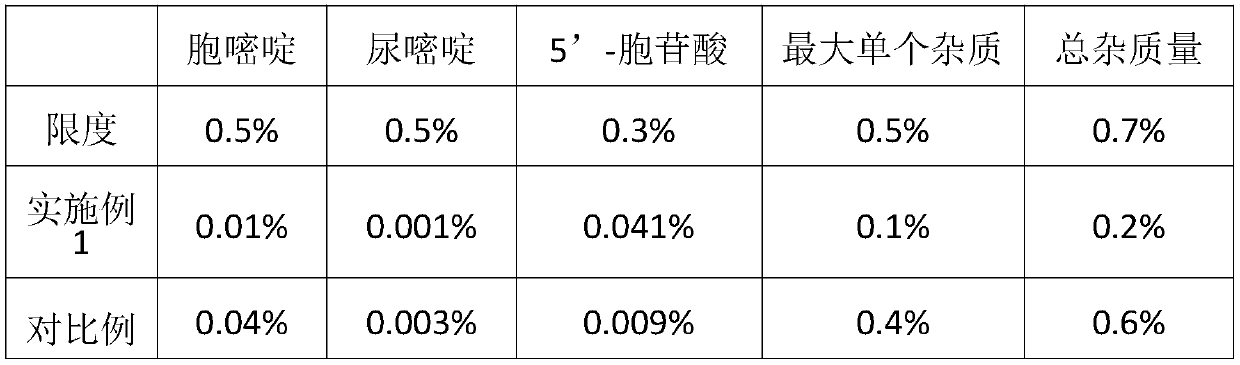
Examples
Embodiment 1
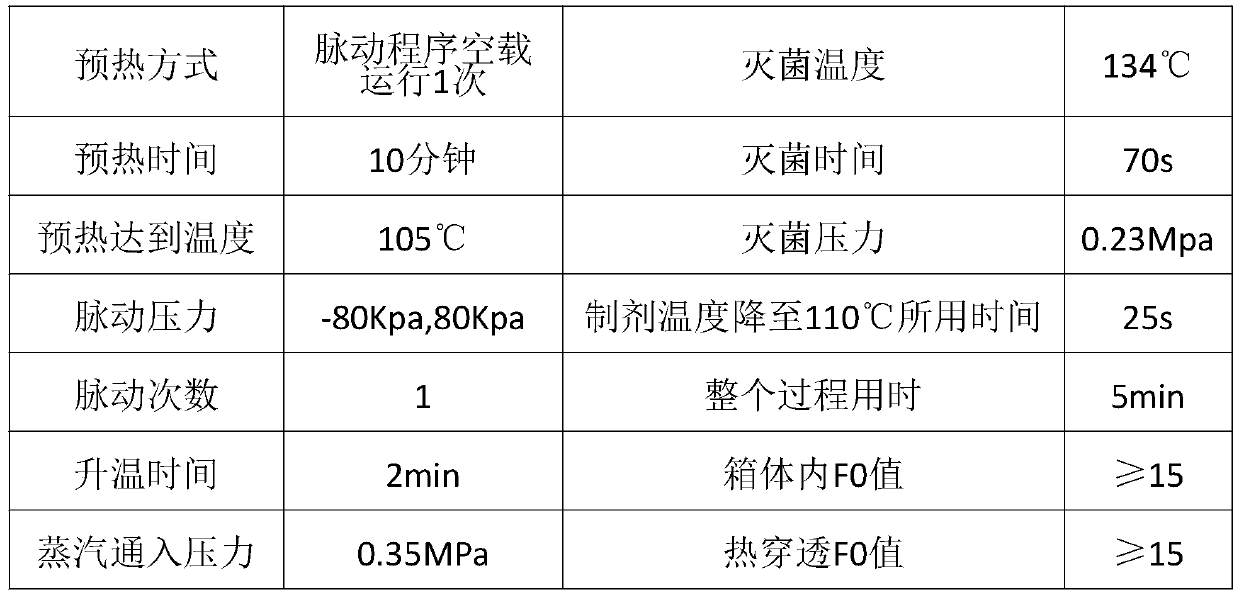
[0043] In the present embodiment, the moist heat sterilization method of the citicoline and its sodium salt injection is a pulsating vacuum sterilization procedure, which specifically includes the following steps:
[0044] S101. Perform no-load preheating on the pulsating vacuum sterilizer; as a preferred implementation of this embodiment, the no-load preheating is performed by pulsating or passing steam into the interlayer; the no-load preheating The box temperature is 100°C-121°C.
[0045] S102, open the box door, put the preparation into the pulsating vacuum sterilizer, and close the box door;
[0046] S103, perform pulsation or replacement; as a preferred implementation of this embodiment, the number of pulsation programs is set to 1-3 times, the replacement time is set to 1s-600s, the upper limit of the pulsation pressure is set to 200Kpa, and the lower limit of the pulsation pressure is set to -95Kpa.
[0047] S104, passing saturated pure steam into the box of the puls...
Embodiment 2
[0057] In this embodiment, the moist heat sterilization method of the citicoline and its sodium salt injection is a steam-air mixture sterilization procedure, which specifically includes the following steps:
[0058] S201. Perform no-load preheating of the mixed steam-air sterilizer; as a preferred implementation of this embodiment, the no-load preheating is performed by pulsating or passing steam into the interlayer; the no-load The temperature of the preheated box is 100°C-121°C.
[0059] S202, open the box door, put the preparation into the mixed steam-air sterilizer, and close the box door;
[0060] S203, passing saturated pure steam into the mixed steam-air box; as a preferred implementation of this embodiment, the saturated pure steam is passed into the box of the sterilizer through a single or multiple pipelines, and the steam The inlet pressure is 0.1Mpa-2.0Mpa, and the heating time is 0.1min-20min;
[0061] S204. Mix and circulate the steam and the air in the mixed ...
Embodiment 3
[0069] In this embodiment, the moist heat sterilization method of the citicoline and its sodium salt injection is a superheated water sterilization procedure, which specifically includes the following steps:
[0070] S301. Preheating the high-pressure superheated water spray sterilizer; as a preferred implementation of this embodiment, in this embodiment, preheating is performed by means of a pulsation program or steam into the interlayer, and the temperature of the preheating box is Raise to 100-121°C, quickly raise the heat penetration temperature of the full load of medicines to close to the sterilization temperature.
[0071] S302. Fix the preparation on the tray and put it into the high-pressure superheated water spray sterilizer, and close the box door;
[0072] S303, passing the sterilizing water into the storage tank of the high-pressure superheated water spray sterilizer, and directly heating the sterilizing water to the sterilizing temperature through a heat exchange...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com