Nicotine salts and methods of making and using same
A technology of nicotine salt and nicotine, which is applied in the fields of tobacco, application, and treatment of tobacco, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
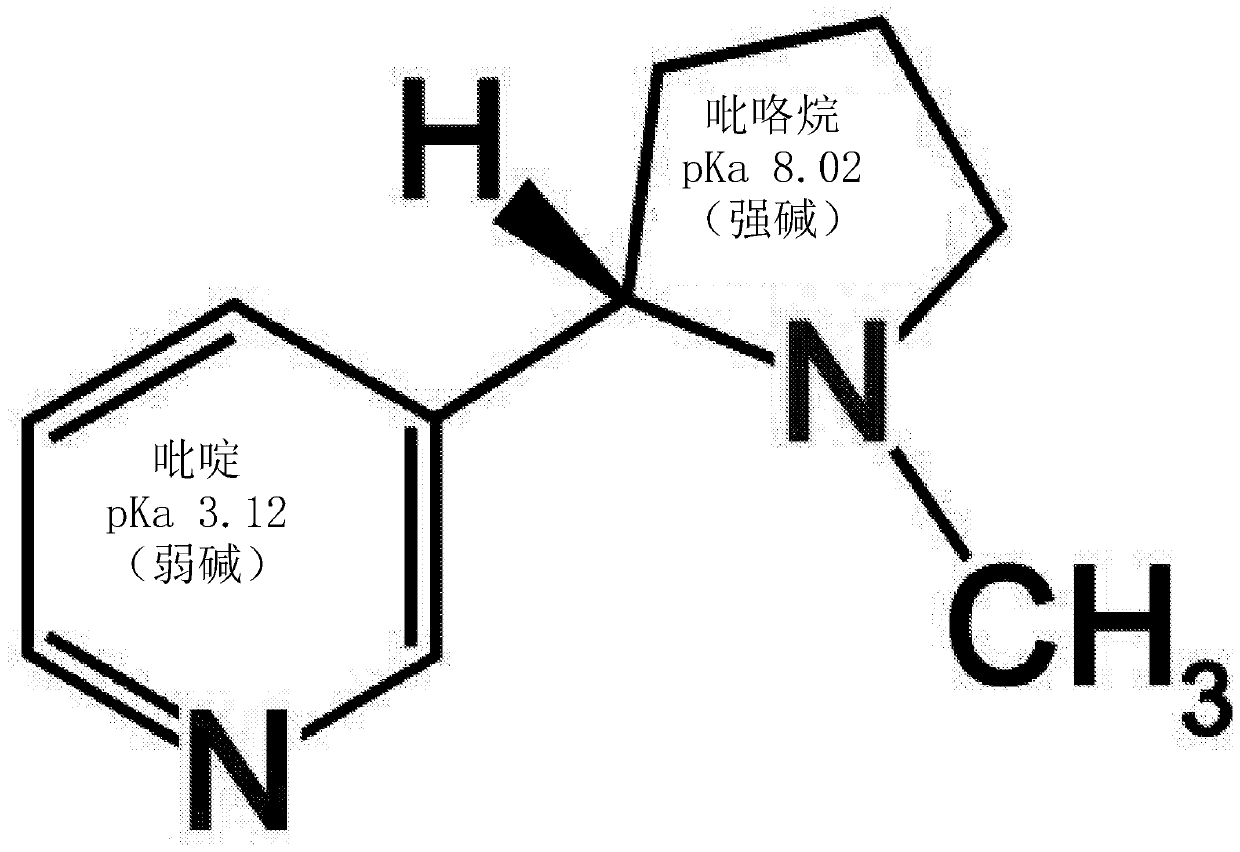
[0053] 2) In certain embodiments herein, different nicotine-organic acid salts have different properties. While free base nicotine has a different and characteristic "flavor" and "throat feel" or "throat hit", nicotine salts provide additional and in some embodiments all have pleasant positive effects. Different user experience. Specific formulations have the potential to favorably alter the user's vaping experience compared to free base nicotine, whether by smoothing, such as reducing the "throat hit" or throat sensation, or by increasing the amount of throat "tingle". features, and in some respects, depends on user preferences. These methods can be modified, changed and / or manipulated in certain embodiments, for example by using specific organic acids to bind to certain centers of nicotine in an orderly fashion (i.e., one or both rings of nicotine Electronegative center of nitrogen). This combination can be controlled by the chemist or manufacturer by embodiments of the i...
Embodiment approach
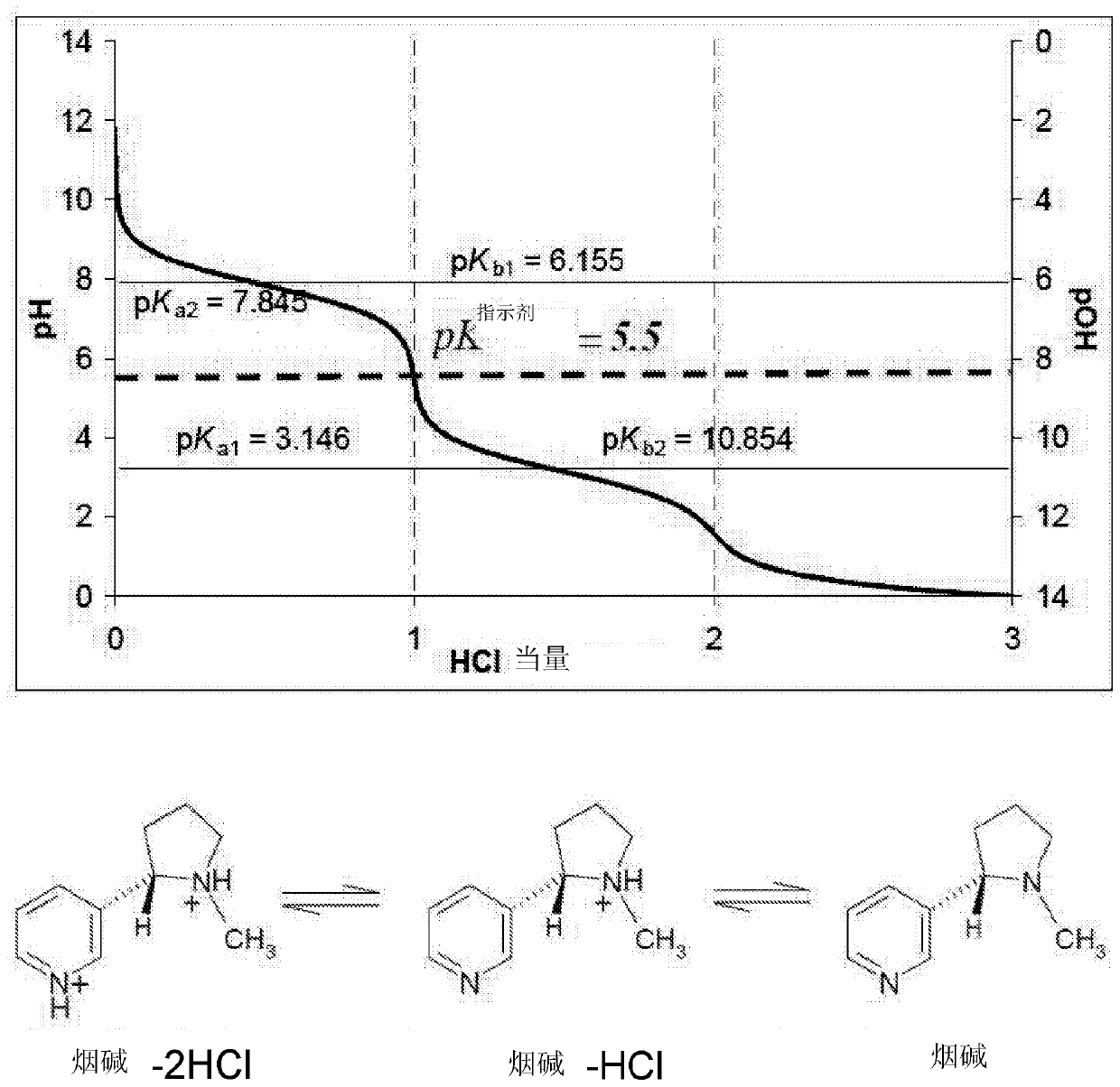
[0099](1) The type of nicotine salt complex selected can have an impact on the overall effect of depositing nicotine onto the mucous membrane of the alveoli in the lung. Preferably, the nicotine salt complex between pH 6-7 is delivered to the lungs with the target environment closest to the alveolar mucosa. Once not bound to nicotine, the alkali-buffering action of organic acids is critical to counteract the alkali onslaught to cells lining the airways. This action would further enhance nicotine diffusion without shock to exposed mucosal cells, resulting in more efficient diffusion of nicotine into surrounding capillaries. Nicotine salt complexes between pH 5-6 will optionally be partially deposited in the alveolar pulmonary mucosa and possibly also along the pharynx of the upper airway. For this application, it is preferred to choose a nicotine salt complex with a higher acidic character.
[0100] (2) The perception to the user can also be controlled by the pH of the overal...
Embodiment I
[0149] Example I: Identification of preferred ketoacid-nicotine salt complexes
[0150] It was determined experimentally which of these embodiments are preferred over others for the ketoacid-nicotine salt complexes described herein. Complexes are listed in descending order according to the following criteria:
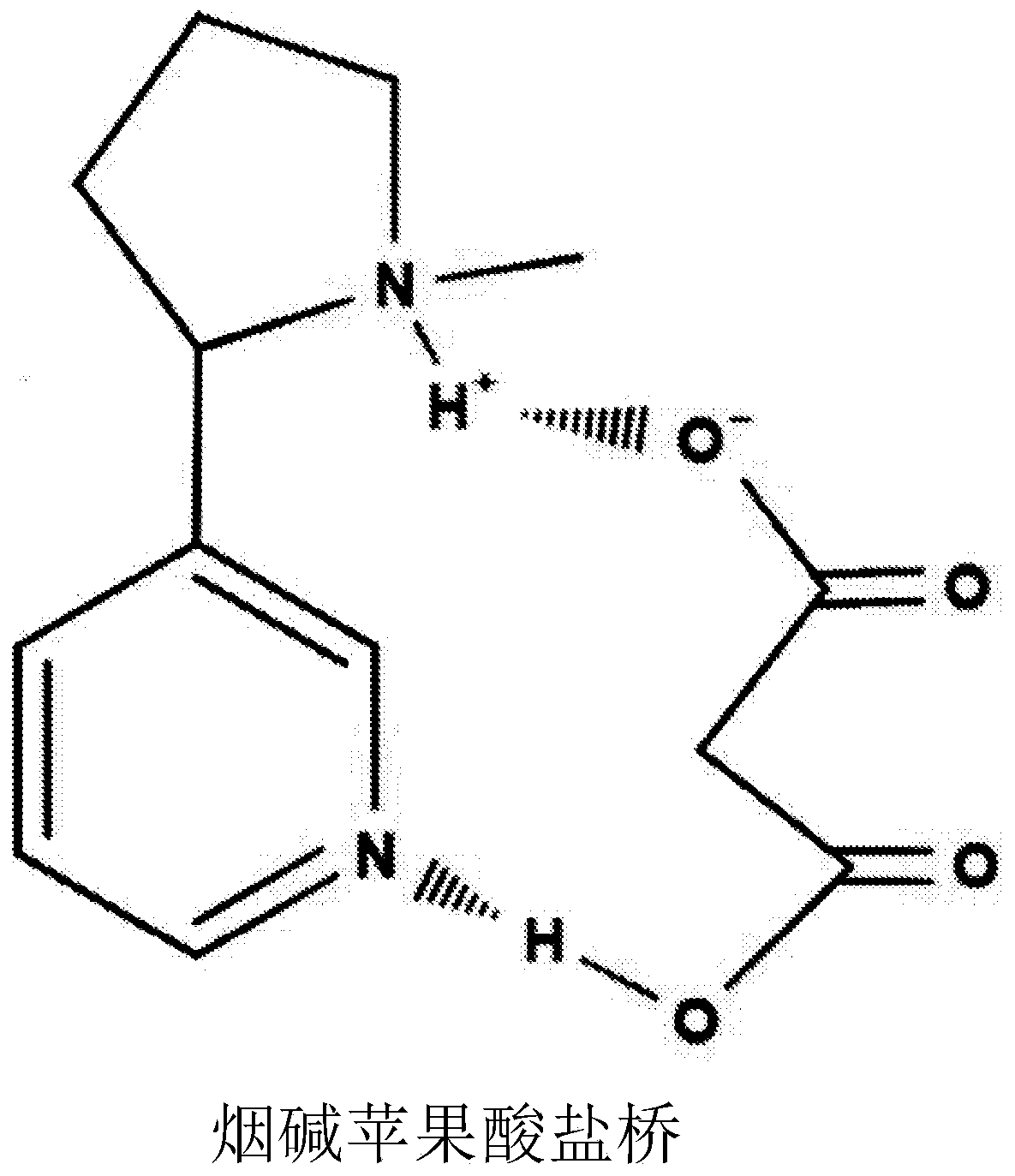
[0151] (a) Bridging and higher order bonds: Preferably, one carboxylic acid function is located on one end of the molecule while at least one keto function is located on the other. The bridged ketoacid nicotine salt complex requires a 2-3 carbon spacing between the keto functional group and the carboxylic acid functional group for bridging to occur.
[0152] (b) Steric hindrance: Excessive molecular size is not desirable for preferred embodiments of the invention.
[0153] (c) Number of carbons relative to functional groups: lesser amount of carbon on the molecule and lesser amount of excess functional groups (other than the required keto and -carboxylate functional...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com