SARS-CoV-2 yolk neutralizing antibody IgY spray for preventing and treating new coronal pneumonia
A sars-cov-2, spray technology, applied in the field of epidemic prevention, can solve the problems of increased virus fatality rate, less plasma source, and difficult to meet the treatment of large patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
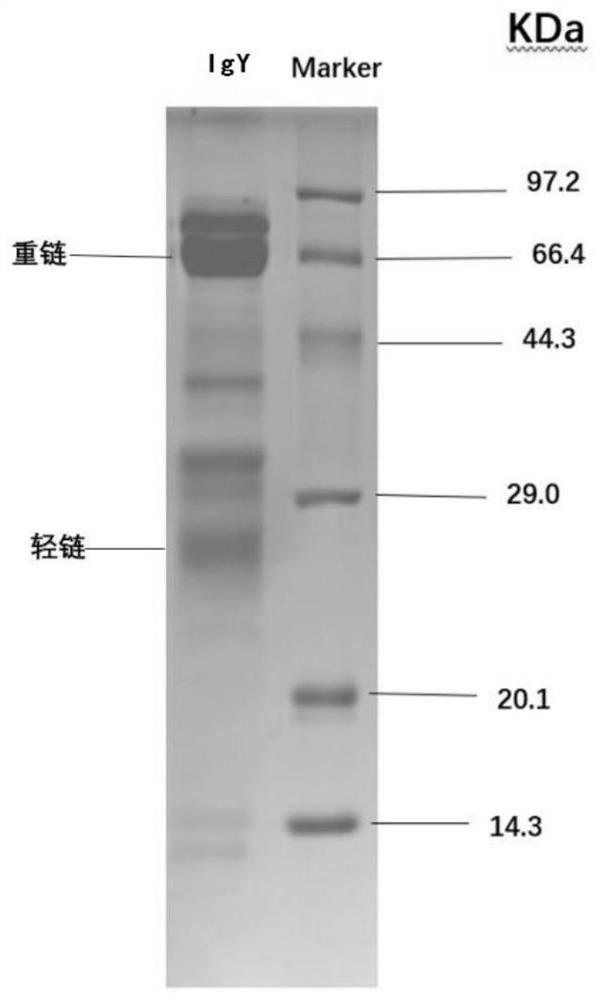
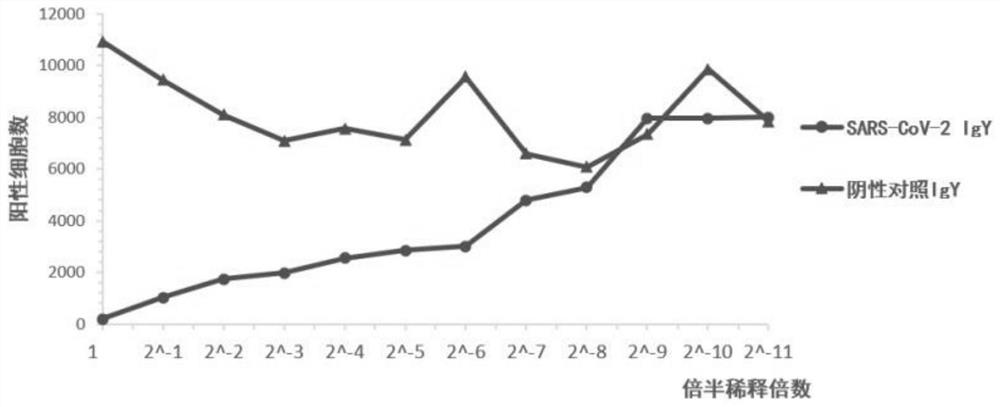
[0039] Example 1 Preparation of yolk neutralizing antibody IgY against novel coronavirus
[0040] 1. Immune laying hens with the RBD protein of the new coronavirus
[0041] Use the new coronavirus RBD protein (see SEQ ID NO.1 for its amino acid sequence) as the antigen, take 200 μg of the antigen each time, add an adjuvant suitable for immunizing chickens, antigen:adjuvant ISA 70=30:70, emulsification, volume ratio, Intramuscular injection of 0.5ml was carried out to laying hens, immunized 3 times, immunized at 3 points, and the interval between each time was 28 days.
[0042] 2. Extract and purify IgY antibody
[0043] Use egg yolk sieves to separate egg yolks from immune hens, add 9 times distilled water and stir to prepare egg yolk liquid, adjust pH to 5.2 with 1MolHCl, set aside for 5 hours at 4°C, centrifuge at 8000rpm for 20 minutes, discard the precipitate, and collect the supernatant. Slowly add n-octanoic acid dropwise to 1% to remove impurity protein and lecithin i...
Embodiment 2
[0056] The preparation of embodiment 2 new coronavirus egg yolk neutralizing antibody IgY spray:
[0057] The ingredients are proportioned according to the following percentages by weight: yolk neutralizing antibody IgY 0.3%, glycerin 10%, NISIN 0.02%, lysozyme 0.5%, chlorhexidine 0.02%, add deionized water to make up 100ml, Use Pasteur disinfection method to inactivate the virus, use 0.22μm membrane filter to sterilize, put the solution into a small portable spray tank and spray it for nasal spray, oral spray prevention, atomization emergency treatment or Emergency injection therapy.
Embodiment 3
[0058] Example 3 Preparation of Egg Yolk Neutralizing Antibody IgY Eye Drops for Prevention of New Coronary Pneumonia
[0059] The ingredients are proportioned according to the following weight percentages: yolk neutralizing antibody IgY of Example 1 0.3%, lysozyme 0.5%, NISIN 0.02%, EDTA-2Na 0.05%, NaCl 0.034%, benzalkonium 0.02%, Glycerin 5%, sorbitol 5%, water-soluble peppermint flavor 0.025%, disodium hydrogen phosphate 0.786%, sodium dihydrogen phosphate 1.04%, deionized water to make up the balance.
[0060] Viruses were inactivated by pasteurization and sterilized by filtration through a 0.22 μm membrane. Put the solution into a small carry-on eye drop bottle for eye drops.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com