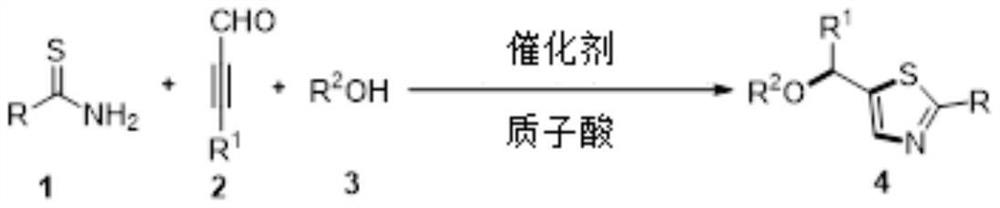
A kind of method and application of functionalized thiazole heterocyclic compound prepared by cu(i) catalyzed multi-component cyclization reaction
A technology for heterocyclic compounds and cyclization reactions, applied in botany equipment and methods, applications, organic chemistry, etc., can solve problems such as harsh conditions and narrow substrate application range, and achieve simple and easy operation and wide substrate application range , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
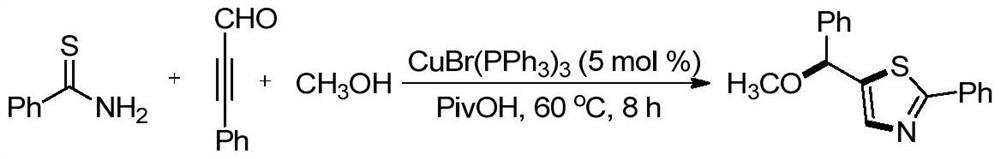
Embodiment 1
[0027] 41.2 mg (0.3 mmol) thiocyanamide, 58.5 mg (0.45 mmol) of aldehyde was added to 10 ml of a reaction bottle with a stirrer, and 3 ml (0.033 m) methanol was added at room temperature, and then 14.0 mg (0.015 mmol) catalyst CUbr (PPH 3 ) 3 And 30.6 mg (0.3 mmol) of specialty acid, stirred at 60 ° C for 8 hours, and the target product was separated by silica gel chromatography at 60 ° C, and the yield was 79%.
[0028]
[0029] Thiazole heterocyclic compound 1 H NMR, 13 C NMR, HR-ESI-MS spectrum data is as follows:
[0030] 1 H NMR (400MHz, CDCL 3 : Δ7.89 (D, J = 3.5 Hz, 2H), 7.59 (S, 1H), 7.45-7.37 (M, 8H), 5.51 (S, 1H), 3.42 (S, 3H).
[0031] 13 C NMR (101MHz, CDCL 3 : Δ169.3, 150.0, 141.2, 140.5, 133.5, 133.1, 131.6, 130.3, 129.3, 129.2, 129.1, 128.9, 128.5, 127.2, 127.1, 126.8, 126.5, 79.5, 57.1.
[0032] HR-MALDI-MS: M / Z CALCD.FOR C 17 Hide 15 NOS [M + H] + : 282.0947, Found: 282.0944.
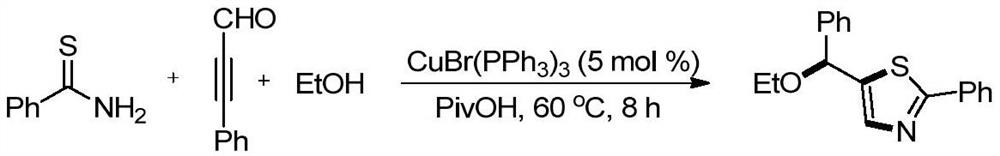
Embodiment 2
[0034] 41.2 mg (0.3 mmol) thiocyanlamide, 58.5 mg (0.45 mmol) aconene was added to a reaction bottle with a stirrer, and 3 ml (0.033 m) ethanol was added at room temperature, and then 14.0 mg (0.015 mmol) catalyst CUbr (PPH 3 ) 3 And 30.6 mg (0.3 mmol) of specialty acid, stirred at 60 ° C for 8 hours, and the target product was separated by silica gel chromatography after drying. The yield was 77%.
[0035]
[0036] Thiazole heterocyclic compound 1 H NMR, 13 C NMR, HR-ESI-MS spectrum data is as follows:
[0037] 1 H NMR (400MHz, CDCL3): Δ7.89 (D, J = 9.8 Hz, 2H), 7.59 (S, 1H), 7.47-7.32 (M, 8H), 5.63 (S, 1H), 3.63-3.54 (M 2H), 1.29 (T, J = 7.0 Hz, 3H).
[0038] 13 C NMR (101 MHz, CDCL3): δ169.0, 141.7, 141.2, 133.8, 130.1, 129.0, 128.8, 128.3, 126.7, 126.5, 77.6, 64.8, 15.4.
[0039] HR-MALDI-MS: M / Z CALCD.FOR C 18 Hide 17 NOS [M + H] + : 296.1104, Found: 296.1104.
Embodiment 3
[0041] 41.2 mg (0.3 mmol) thiosamide, 58.5 mg (0.45 mmol) aldehyde substrate was added to a reaction bottle with stirred sub-reaction, and 3 ml (0.033m) isopropyl alcohol was added at room temperature, and then 14.0 mg (0.015) Mmol) Catalyst CUbr (PPH 3 ) 3 And 30.6 mg (0.3 mmol) of specialty acid, stirred at 60 ° C for 8 hours, and the target product was separated by silica gel chromatography after drying, and the yield was 74%.
[0042]
[0043] Thiazole heterocyclic compound 1 H NMR, 13 C NMR, HR-ESI-MS spectrum data is as follows:
[0044] 1 H NMR (400 MHz, CDCL3): Δ7.89 (D, J = 3.9 Hz, 2H), 7.55 (S, 1H), 7.46-7.36 (M, 8H), 5.74 (S, 1H), 3.79-3.73 (M 1H), 1.24 (DD, J = 14.6, 6.1 Hz, 6h).
[0045] 13 C NMR (101MHz, CDCL3): Δ169.0, 141.5, 141.0, 133.8, 130.1, 129.0, 128.7, 128.2, 126.8, 126.5, 74.8, 69.8, 22.5, 22.2.
[0046] HR-MALDI-MS: M / Z CALCD.FOR C 19 Hide 19 NOS [M + H] + : 310.1260, Found: 310.1256.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com