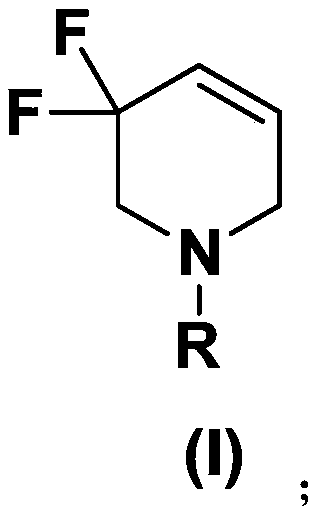
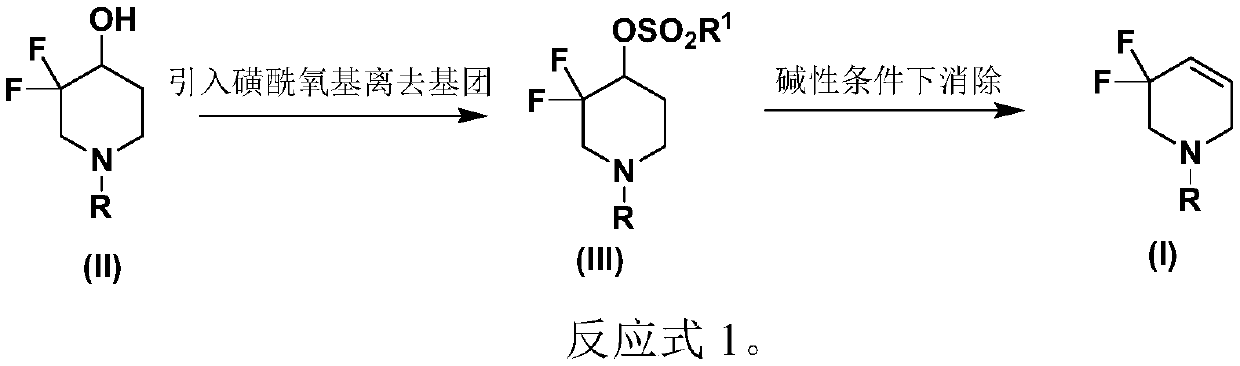
3,3-difluoro-1,2,3,6-tetrahydropiperidine derivative and preparation method thereof
A technology of tetrahydropiperidine and derivatives, which is applied in the field of 3,3-difluoro-1,2,3,6-tetrahydropiperidine derivatives and their preparation, and can solve the problems of no reported difluoro structure and the like , to achieve the effect of high yield, convenient industrial production operation, and controllable environmental impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Preparation of N-tert-butoxycarbonyl-3,3-difluoro-4-trifluoromethylsulfonyloxypiperidine (V)
[0045]
[0046] Under nitrogen protection, the compound N-tert-butylcarbonyl-3,3-difluoro-4-hydroxypiperidine (100 g, 0.42 mol, 1.00 eq) represented by formula (IV) was dissolved in 800 mL of dichloromethane, and pyridine ( 100.0g, 1.26mol, 3.00eq), the mixed solvent was cooled to minus 40°C, and trifluoromethanesulfonic anhydride (177.7g, 0.63mol, 1.50eq) was slowly added. The obtained pale yellow clear liquid was slowly returned to room temperature, and the reaction was monitored for completion after continuous stirring at room temperature for 2 hours. The reaction solution was quenched by adding 600 mL of water, and the layers were separated. The separated organic phase was washed with a saturated laboratory once, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure to obtain 155.0 g of a light yellow solid. The...
Embodiment 2
[0049] Example 2: Preparation of N-benzyl-3,3-difluoro-4-trifluoromethylsulfonyloxypiperidine (VII)
[0050]
[0051] Under nitrogen protection, the compound N-benzyl-3,3-difluoro-4-hydroxypiperidine (100 g, 0.44 mol, 1.00 eq) represented by formula (VII) was dissolved in 800 mL of dichloromethane, and pyridine (104.4 g) was added. , 1.32mol, 3.00eq), the mixed solvent was cooled to minus 40°C, and trifluoromethanesulfonic anhydride (186.1g, 0.66mol, 1.50eq) was slowly added. The obtained pale yellow clear liquid was slowly returned to room temperature, and the reaction was monitored for completion after continuous stirring at room temperature for 1.5 hours. The reaction solution was quenched by adding 600 mL of water, and the layers were separated. The separated organic phase was washed with a saturated laboratory once, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure to obtain 159.2 g of a light yellow solid. The crude ...
Embodiment 3
[0054] Example 3: Preparation of N-methyl-3,3-difluoro-1,2,3,6-tetrahydropiperidine (X)
[0055]
[0056] Under nitrogen protection, the compound N-methyl-3,3-difluoro-4-hydroxypiperidine (10 g, 66.2 mmol, 1.00 eq) represented by formula (X) was dissolved in 100 mL of dichloromethane, and pyridine (28.0 g) was added. , 198.6mmol, 3.00eq), the mixed solvent was cooled to minus 40°C, and trifluoromethanesulfonic anhydride (186.1g, 99.3mmol, 1.50eq) was slowly added. The obtained pale yellow clear liquid was slowly returned to room temperature, and the reaction was monitored for completion after continuous stirring at room temperature for 1.5 hours. The reaction solution was quenched by adding 100 mL of water, and the layers were separated. The separated organic phase was washed with a saturated laboratory once, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure to obtain a light yellow oil. %yield): 1 H NMR (400MHz, CDCl 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com