Production process of tadalafil bulk drug
A tadalafil and production process technology, applied in the field of production process of tadalafil API, can solve the problems of complex production process, high market price, low comprehensive yield, etc., achieve low cost and reduce production cost , the effect of high purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
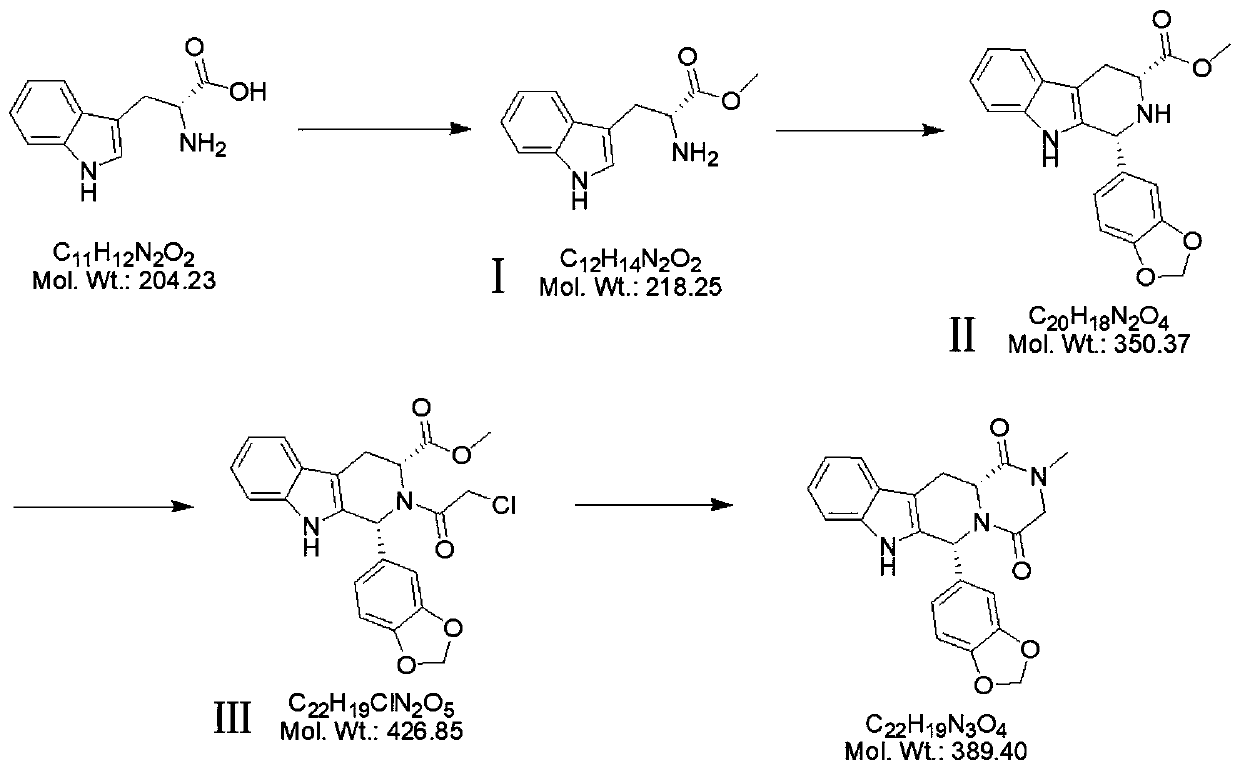
[0019] A kind of production technology of tadalafil crude drug, such as figure 1 , including the following steps:
[0020] A1: Esterification reaction between methanol and D-tryptophan to obtain intermediate Ⅰ;
[0021] A2: intermediate Ⅰ undergoes condensation reaction with piperonal to obtain intermediate Ⅱ;
[0022] A3: intermediate Ⅱ undergoes acylation reaction with chloroacetyl chloride to obtain intermediate Ⅲ;
[0023] A4: The intermediate III undergoes a cyclization reaction with monomethylamine to obtain the raw material drug of tadalafil.
[0024] In this embodiment, step A1 is: put 50.0kg of methanol and 12.0kg of D-tryptophan into the reaction kettle, drop the temperature to 10-20°C and add 16kg of thionyl chloride dropwise; Stir and react at 45°C for 2 hours; after the reaction, concentrate under reduced pressure and distill out methanol, put 50.0kg of ethyl acetate into the reaction kettle after steaming, beat at room temperature for 1 hour, then spin filter,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com