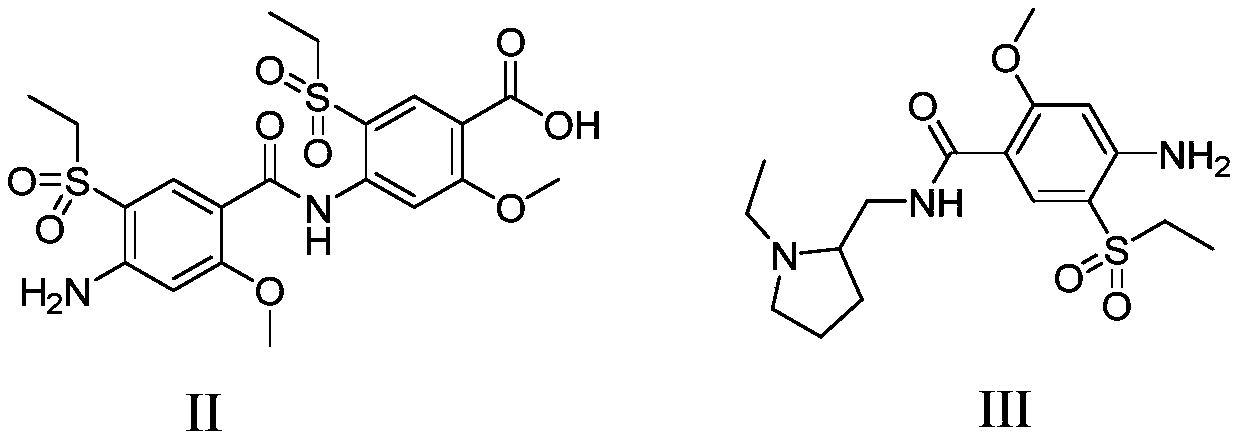
Amisulpride impurity and preparation method thereof
A technology for amisulpride and impurities, which is applied in the field of amisulpride impurities and its preparation, can solve the problems that the synthesis method has no literature reports, etc., and achieve the effects of simple operation, short reaction route and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
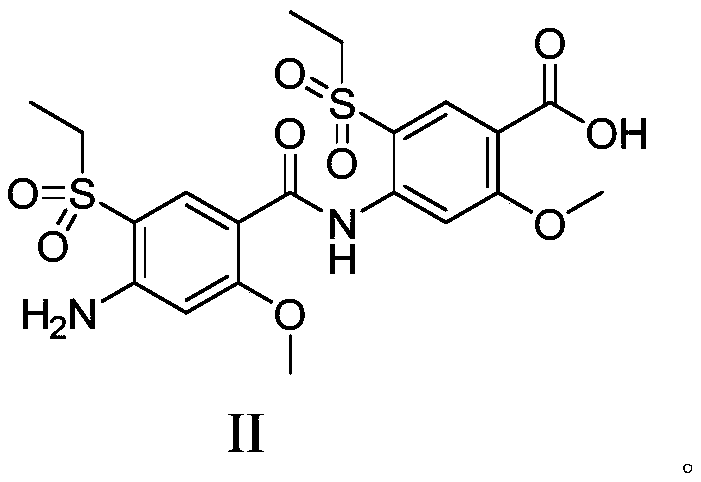
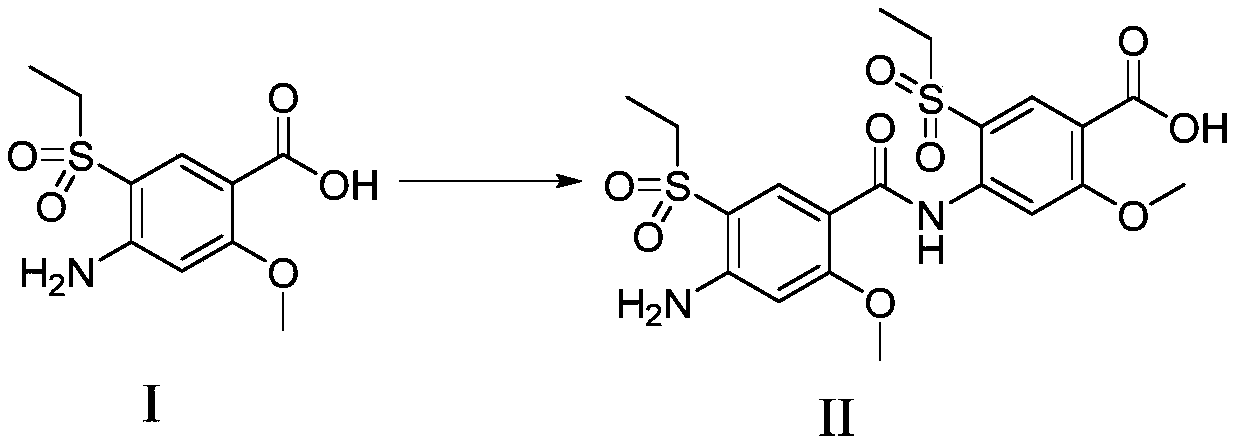
[0035] Add 4-amino-5-(ethylsulfonyl)-2-methoxybenzoic acid (Compound I) (8.0g, 30.84mmol) and N,N-dimethylformamide (30ml) into a 50mL three-necked flask , stirring to dissolve, adding 4-dimethylaminopyridine (1.88g, 15.4mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (2.96g, 15.4mmol) successively , heated to 25-40°C, stirred for 12h, added water (300ml), stirred for 1h, solid precipitated, filtered to obtain a light yellow solid. The light yellow solid was stirred and dissolved with ethyl acetate (300ml), washed with water (100ml×2), washed with 1mol / L hydrochloric acid (100ml), washed with saturated aqueous sodium bicarbonate (100ml), washed with saturated brine (100ml) Wash, dry over anhydrous sodium sulfate, filter, concentrate under reduced pressure at 40°C to obtain 10 g of crude product, add ethyl acetate (100ml) to the crude product, heat up to 60-70°C and stir for 2-3 hours, then cool down to 5-10°C and stir for 2-3 hours , a large amount of solid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com