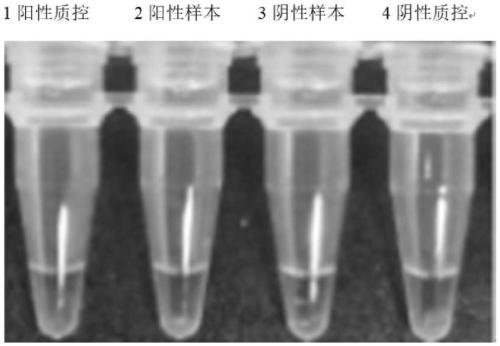
Closed SARS-CoV-2 isothermal amplification nucleic acid detection kit
A sars-cov-2, kit technology, applied in DNA/RNA fragments, recombinant DNA technology, microbial determination/inspection, etc., can solve the problem that it is difficult to achieve real-time detection of new coronavirus pneumonia on-site and without nucleic acid extraction. steps, unfavorable rapid detection and other problems, to avoid secondary pollution, low detection cost, and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] This embodiment provides a closed SARS-CoV-2 isothermal amplification nucleic acid detection kit, the kit includes reaction premix A, reaction premix B, HNB chromogen, detection tube, standard positive template, negative control. The reaction premix consists of reaction premix A and reaction premix B. Wherein, the reaction premix A includes Bst DNA large fragment polymerase, AMV reverse transcriptase; the reaction premix B includes 10X reaction buffer, primer set, dNTPs, magnesium sulfate, betaine, 6wt% formamide and DEPC water.
[0051] The primer set includes a pair of outer primers F3-1 and B3-1, a pair of inner primers FIP-1 and BIP-1, and a pair of loop primers LF-1 and LB-1.
[0052] The nucleotide sequences of F3-1, B3-1, FIP-1, BIP-1, LF-1, LB-1 are as follows:
[0053] F3-1 (SEQ ID NO.1): 5'CTAGGTTTCAAACTTTACTTGC3';
[0054] B3-1 (SEQ ID NO.2): 5'CCTTTTTCTACAGTGAAGGATT3';
[0055] FIP-1 (SEQ ID NO.3):
[0056] 5'CACATAATAAGCTGCAGCACCA-TACATAGAAGTTATTTGACTCC...
Embodiment 2
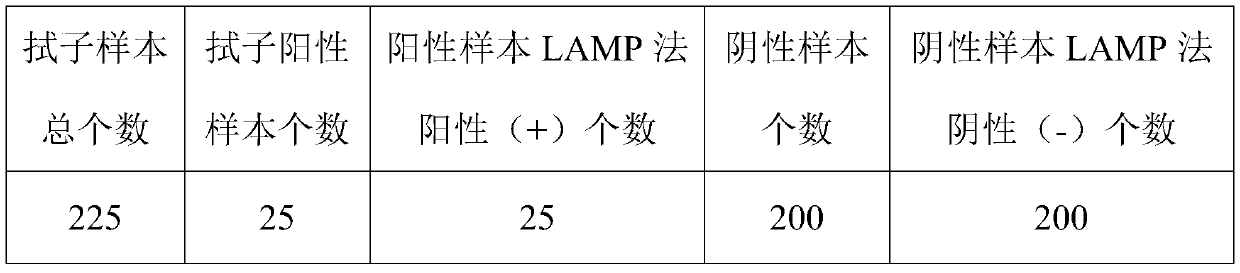
[0071] In combination with the requirements of the detection standards detected by the kit of the present invention, clinical verification has been carried out on the samples to be tested.
[0072] Comparison of LAMP test results with qPCR test
[0073]
[0074] LAMP positive detection rate for positive samples: 100%
[0075] LAMP positive detection rate for negative samples: 100%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com