Biosensor for detecting tumor markers
A technology of biosensors and tumor markers, applied in the direction of biological testing, biomaterial analysis, biochemical equipment and methods, etc., can solve the problems of limiting the practical application of colorimetric pH sensing, low target abundance, etc., to overcome the low detection Abundance targets, removal of interference from complex components, effectiveness of simplified detection methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
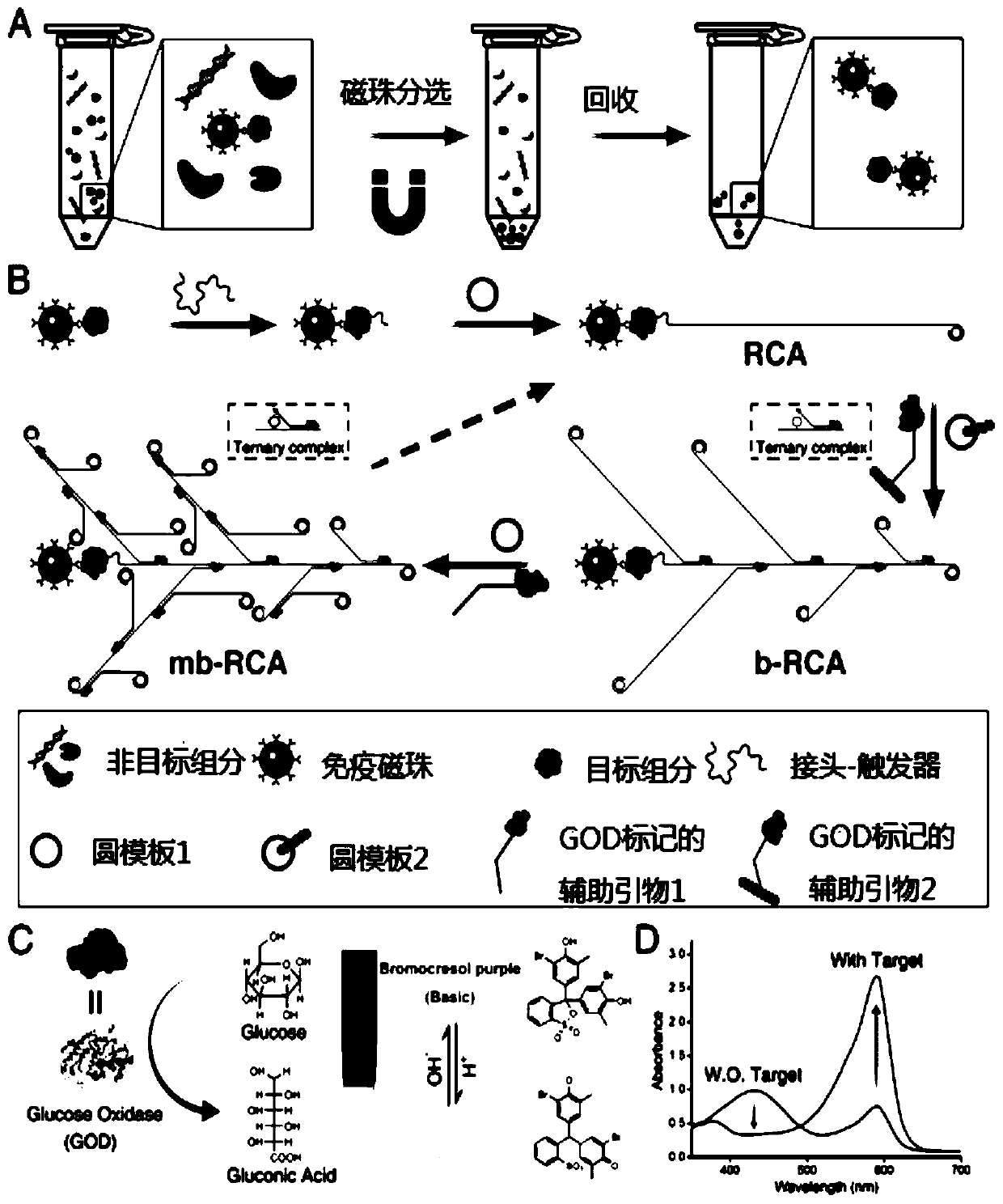
[0038] Preparation process and test method
[0039] A biosensor for detecting tumor markers is made by the following steps:
[0040] I. Use immunomagnetic beads to selectively isolate targets;
[0041] II. Bind the aptamer-trigger to the target to form a sandwich structure of immunomagnetic beads / target / aptamer-trigger;
[0042] III. The aptamer-trigger immobilized on the surface of the immunomagnetic beads initiates linear rolling circle amplification in the presence of circular template 1 to generate a product of linear rolling circle amplification;
[0043] IV. The GOD-labeled auxiliary primer 2 and the circular template 2 combine with the product of the linear rolling circle amplification in step III to form a ternary complex, and the auxiliary primer 2 starts the branched rolling circle amplification to generate branched products;
[0044] V. GOD-labeled auxiliary primer 1 and circular template 1 combine with the branched product in step IV to form another ternary compl...
experiment example
[0057] Experimental example: Feasibility verification and optimization comparison of colorimetric pH sensing.
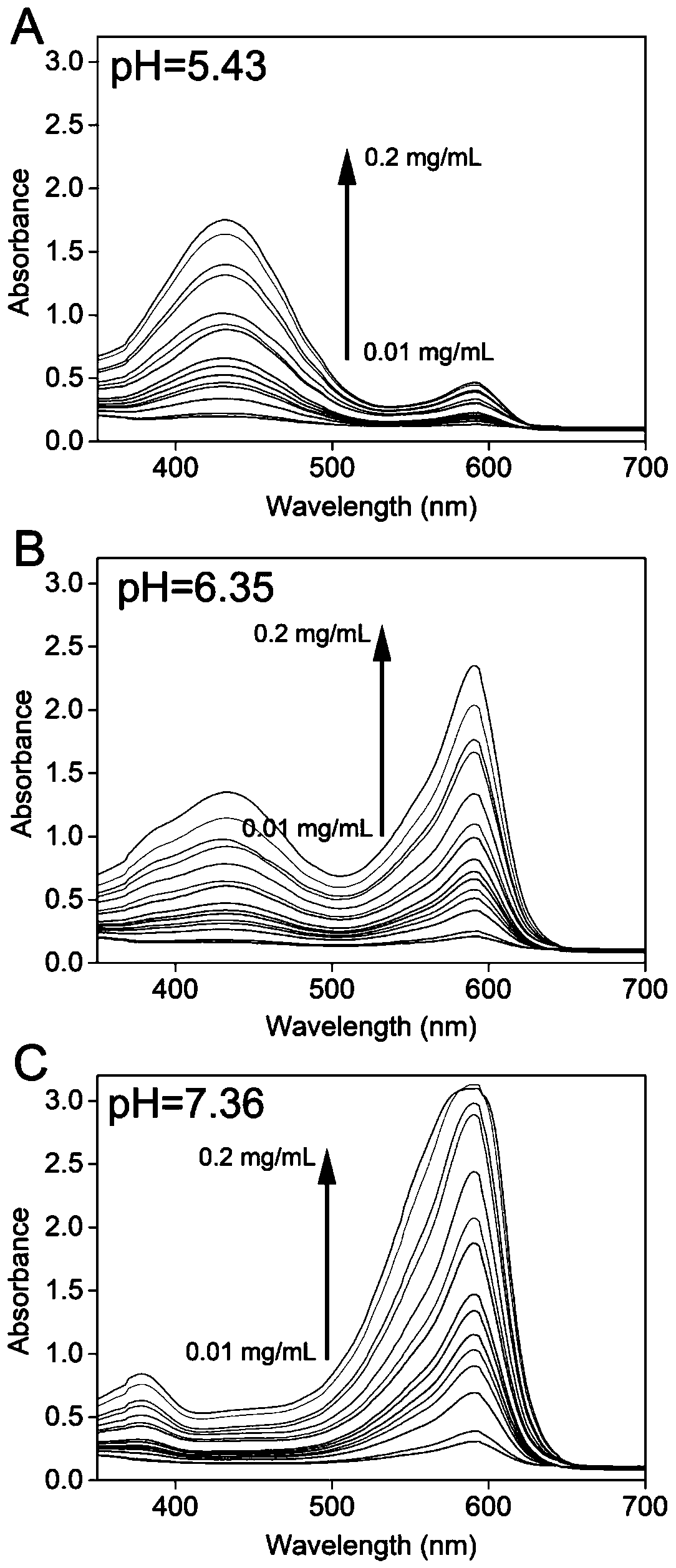
[0058] The GOD selected by the present invention works well under neutral pH value (5.2~6.8), consistent with the range of discoloration of BCP (see appendix figure 2 A, B and attached image 3 ).
[0059] Further, neutral pH 7.0 was selected as the initial pH for further experiments, because the color change from neutral pH to acidic pH is obvious to the naked eye. attached figure 2C shows that even at a low GOD concentration (2 nM), the change from purple to yellow can be observed with the naked eye in a short time (30 min), indicating that GOD performs well in pH sensing.
[0060] attached figure 2 D provides more accurate colorimetric results. It can be seen that the A590 nm / A430 nm of all the positive samples (with GOD) had obvious changes, while the A590 nm / A430 nm of the negative samples (without GOD) had little change. The above results demonstrate t...
Embodiment 3
[0062] Experimental example: comparison of amplification efficiency of mb-RCA
[0063] as attached Figure 4 As shown, the change of pH was positively correlated with the concentration of GOD. Therefore, enriching more GOD by signal amplification technology is an effective way to improve the detection effect of low-abundance targets. In the present invention, we propose a novel isothermal DNA signal amplification technique called mb-RCA. Since mb-RCA can be divided into two closely related LRCA systems, these two LRCA systems are verified first. as attached Figure 4 As shown in A, LRCA can only be initiated in the presence of a trigger, template and polymerase. Subsequently, the DNA polymerase was optimized (attached Figure 4 B). Among the two commonly used DNA polymerases that can work normally at 37°C, the amplification efficiency of phi29-DNA polymerase is much higher than that of Klenow Fragment (3'-5'exo-) many. In addition, good amplification specificity was ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com