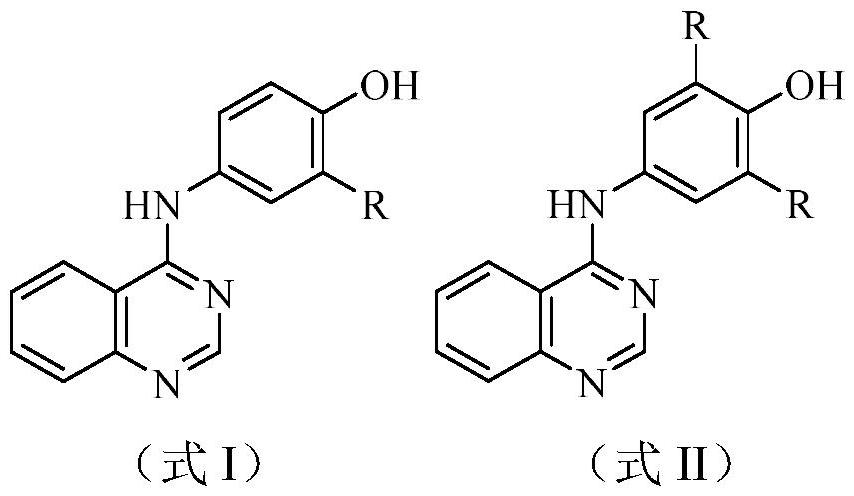
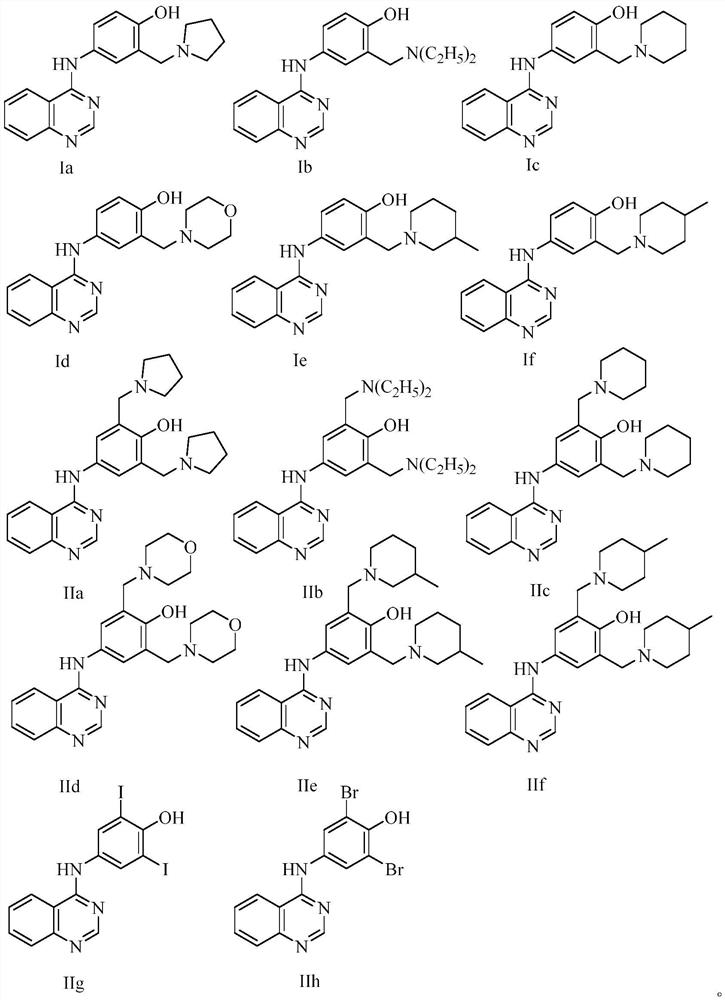
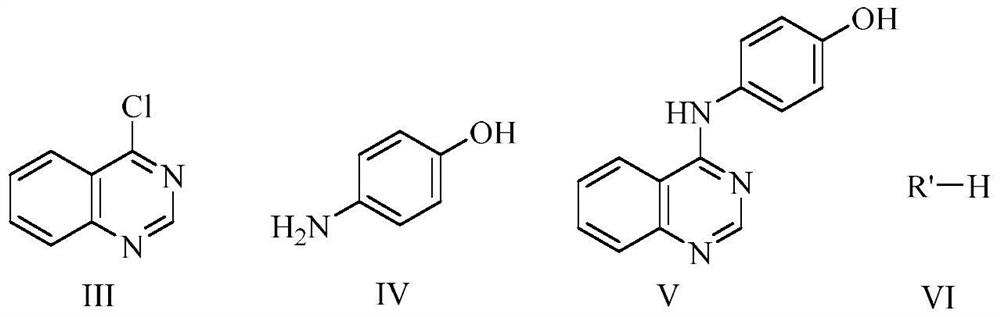
Quinazoline derivatives and their preparation and use
A technology of derivatives and drugs, applied in the field of medicine, to achieve a good effect of preventing and controlling chicken coccidiosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of 4-(3'-pyrrolidinylmethyl-4'-hydroxyanilino)quinazoline (compound Ia)
[0040] Step 1) Synthesis of 4-(p-hydroxyanilino) quinazoline (compound V)
[0041] Add 4-chloroquinazoline III (3.3g, 20.05mmol) and p-aminophenol IV (2.2g, 20.05mmol) into a 250mL three-necked flask, add ethyl acetate (100mL), heat to reflux at 78°C, and react 8h, TLC monitors the reaction, the developer is petroleum ether-ethyl acetate (volume ratio 1:2, 5 drops of methanol is added to every 1mL of ethyl acetate), almost complete. After the reaction was completed and the temperature was lowered to room temperature, suction filtration was performed, and the filter cake was rinsed with a small amount of ethyl acetate. Then put the filter cake into a 250 mL beaker, add 100 mL of saturated sodium bicarbonate solution, stir for 20 min, filter with suction, wash the filter cake with water until neutral, and dry naturally to obtain 3.8 g of off-white pure product with a yield of ...
Embodiment 2
[0046] Example 2 Preparation of 4-(3'-diethylaminomethyl-4'-hydroxyanilino)quinazoline (compound Ib)
[0047] According to the method in Example 1, this example uses intermediate V (when preparing, the reaction condition is 78 ° C under heating and reflux, the reaction time is 8 hours, and the rest of the preparation process is the same as in Example 1) as raw material, and formaldehyde and diethylamine are produced. Compound Ib was obtained by Nisch reaction (the reaction condition was reflux at 78°C, and the reaction time was 36h). The yield was 46.6%.
[0048] The structural confirmation data are as follows:
[0049] Melting point 195°C; 1 HNMR (600MHz, DMSO-d 6)δ9.62(s,1H),8.51–8.46(m,2H),7.82(ddd, J=8.3,6.9,1.3Hz,1H),7.74(dd,J=8.3,1.2Hz,1H),7.59 (ddd, J=8.2,6.8,1.3Hz,1H),7.50(dd,J=8.6,2.6Hz,1H),7.44(d,J=2.6Hz,1H),6.73(d,J=8.6Hz, 1H), 3.75(s, 2H), 2.58(q, J=7.1Hz, 4H), 1.05(t, J=7.1Hz, 6H).
Embodiment 3
[0050] Example 3 Preparation of 4-(3'-hexahydropyridylmethyl-4'-hydroxyanilino)quinazoline (compound Ic)
[0051] According to the method in Example 1, this example uses intermediate V (when preparing, the reaction condition is 75 ℃ heating to reflux, the reaction time is 10h, and the rest of the preparation process is the same as Example 1) as raw material, and formaldehyde, hexahydropyridine Compound Ic was obtained by Mannich reaction (the reaction condition was reflux at 75°C, and the reaction time was 35h). The yield was 72.7%.
[0052] The structural confirmation data are as follows:
[0053] Melting point 226°C; 1 HNMR (600MHz, DMSO-d 6 )δ10.87(s,1H),9.62(s,1H),8.49(d,J=2.7Hz,1H),8.48(d,J=1.3Hz,1H),7.81(ddd,J=8.3,6.9 ,1.3Hz,1H),7.74(dd,J=8.4,1.2Hz,1H),7.58(ddd,J=8.3,6.8,1.3Hz,1H),7.51(dd,J=8.6,2.6Hz,1H) ,7.42(d,J=2.6Hz,1H), 6.75(d,J=8.6Hz,1H),3.63(s,2H),2.46(s,4H),1.54(p,J=5.6Hz,4H) ,1.43(d,J=7.6Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com