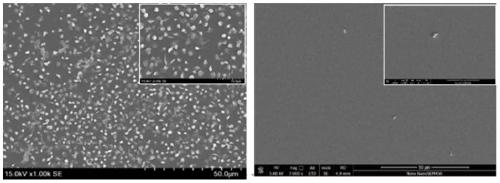
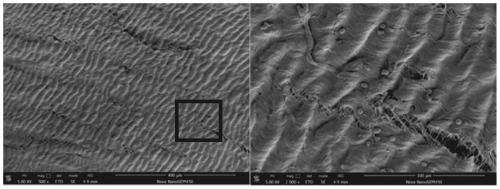
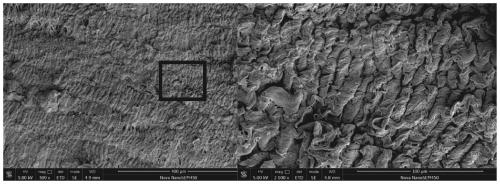
Recombinant human type-III collagen modified biovalve material and preparation method thereof
A collagen and biological valve technology, which is applied in the field of recombinant human type III collagen modified biological valve material and its preparation field, can solve problems such as prone to coagulation, and achieve remarkable anticoagulation performance and low immune rejection in the human body. , the effect of high cell adhesion activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A method for preparing a biological valve material modified by recombinant human type III collagen, comprising the following steps:
[0027] (1) Collect fresh porcine pericardium, clean it, and then soak it in an aqueous glutaraldehyde solution with a volume concentration of 0.6% for 24 hours to obtain an animal-derived pericardium biomaterial crosslinked by glutaraldehyde;
[0028] (2) Wash the animal-derived pericardial biological material cross-linked by glutaraldehyde, soak it in 30 mg / ml recombinant human type III collagen aqueous solution, soak for 24 hours, then take it out and clean it with distilled water; among them, the recombinant human-derived type III collagen The amino acid sequence of type collagen is GERGAPGFRGPAGPNGIPGEKGPAGERGAP.
Embodiment 2
[0030] A method for preparing a biological valve material modified by recombinant human type III collagen, comprising the following steps:
[0031] (1) Collect fresh porcine pericardium, clean it, and then soak it in an aqueous glutaraldehyde solution with a volume concentration of 0.6% for 12 hours to obtain an animal-derived pericardium biomaterial crosslinked by glutaraldehyde;
[0032] (2) Wash the animal-derived pericardial biological material cross-linked by glutaraldehyde and soak it in 15 mg / ml recombinant human type III collagen aqueous solution for 24 hours, then take it out and clean it with distilled water; among them, the recombinant human-derived type III collagen The amino acid sequence of type collagen is GERGAPGFRGPAGPNGIPGEKGPAGERGAP.
Embodiment 3
[0034] A method for preparing a biological valve material modified by recombinant human type III collagen, comprising the following steps:
[0035] (1) Collect fresh porcine pericardium, clean it, and then soak it in an aqueous glutaraldehyde solution with a volume concentration of 0.6% for 12 hours to obtain an animal-derived pericardium biomaterial crosslinked by glutaraldehyde;
[0036] (2) Wash the animal-derived pericardial biological material cross-linked by glutaraldehyde and soak it in 30 mg / ml recombinant human type III collagen aqueous solution for 1 hour, then take it out and clean it with distilled water; among them, the recombinant human-derived type III collagen The amino acid sequence of type collagen is GERGAPGFRGPAGPNGIPGEKGPAGERGAP;
[0037] (3) Soak the material obtained in step (2) in 50mmol / L sodium cyanoborohydride (NaCNBH 3 ) in PBS solution, soaked for 12 hours, and then washed with distilled water.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com