Oncolytic virus vaccine and adoptive immune cell combination therapy
An oncolytic virus and oncolytic technology, applied in the direction of viruses, viral peptides, viruses/bacteriophages, etc., can solve a lot of manpower and material resources and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
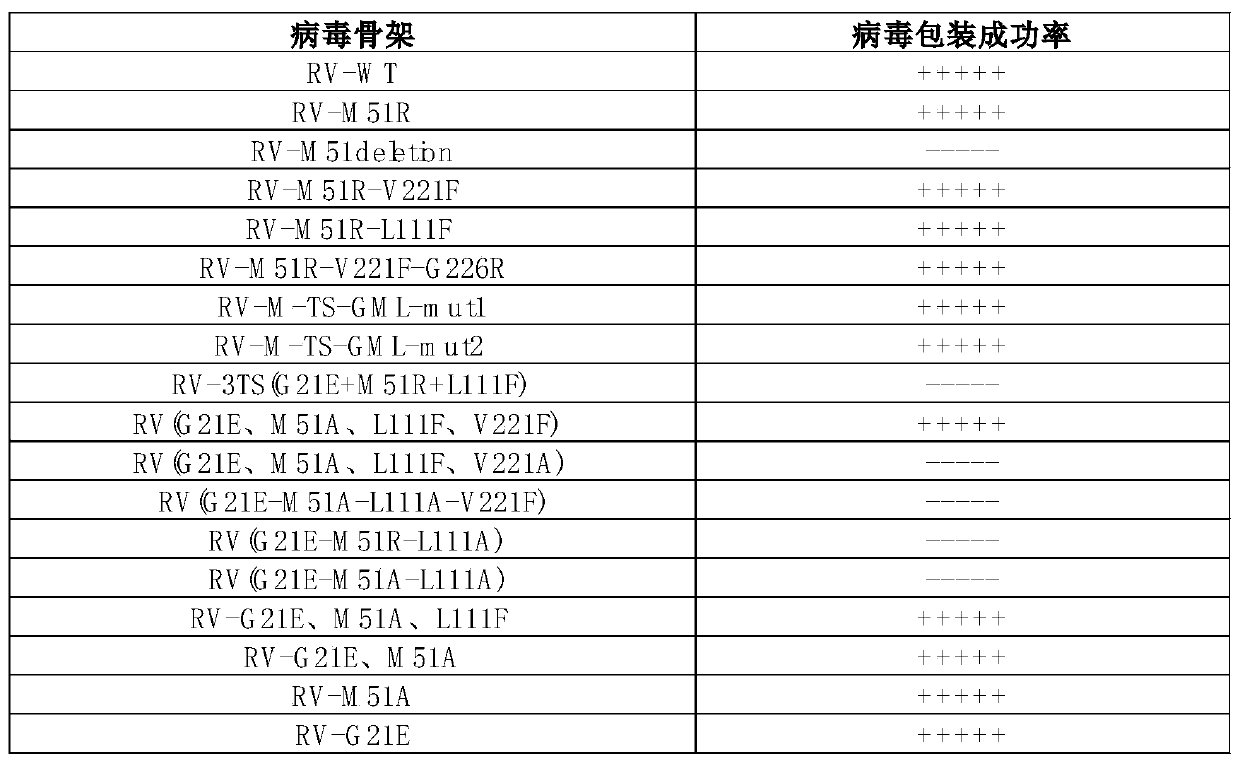
[0224] Example 1: Virus rescue of the attenuated mutant strain (RV-Mut) constructed based on VSV rod-shaped oncolytic virus
[0225] Based on the efficient rescue system of VSV virus, different attenuated strain systems were constructed, and the virus rescue situation of single point and multiple points was randomly mutated. It is further confirmed whether the M protein of the VSV virus can be mutated arbitrarily, and virions can be obtained (that is, whether any mutation can be performed, and the virions can be rescued).
[0226] The concrete steps of above-mentioned virus rescue system construction are as follows:
[0227] 1. Spread BSR-T7 cells in a 6-well plate to make the cell volume reach 4×10 5 1 / well, add vT7 14-16 hours after plating, and transfect after 4 hours of virus infection.
[0228] 2. Use opti-MEM medium to dilute the plasmid. Among them, the total amount of plasmid was 5 μg, and then 7.5 μl PLUS Reagent was added. Dilute 10 μl of Lipofectamine LTX with c...
Embodiment 2
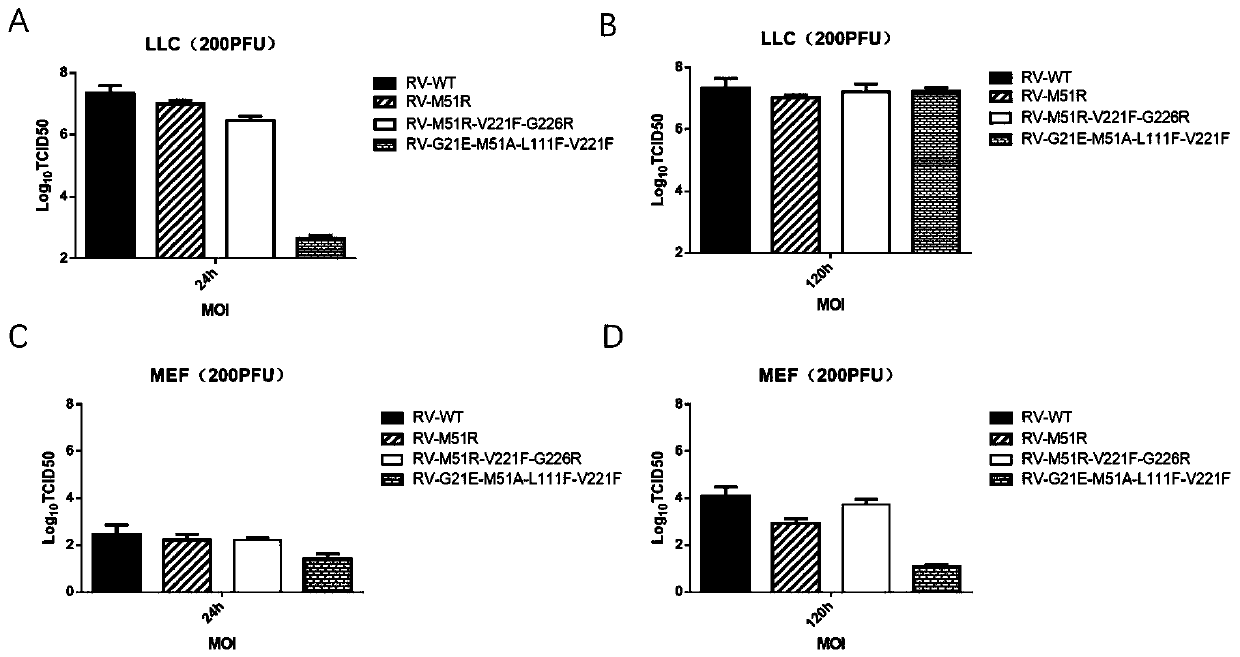
[0236] Example 2: Virus titer detection of different RV-Mut virus strains, virus replication of different attenuated strains including RV-4Mut at different time points in normal cell MEF and tumor cell LLC.
[0237] In the MEF / LLC cell culture medium, add the following viruses: VSV-GFP-WT, RV-GFP-M51R, RV-GFP-M51R-V221F-G226R (RV-3Mut), RV-GFP-G21E-M51A-L111F - V221F (RV-4Mut) each 200PFU, detect the titer (TCID50) of the virus produced by the virus strain.
[0238] The concrete steps of detecting the titer of above-mentioned virus are as follows:
[0239] 1. Add 3 mL of Vero (LLC / Hela) cell suspension to each well of a 6-well culture plate to make the cell volume reach 4×10 5 pcs / well, 6 holes in total, 37°C, 5% CO 2 Cultivate for 16h.
[0240]2. Add 200 PFU each of viruses RV-WT, RV-M51R, RV-M51R-V221F-G226R, RV-G21E-M51A-L111F-V221F to each well, and set 2 wells for normal cell control. Harvest 100 μl of cell supernatant at each time point of 12h, 24h, 48h, 72h, 80h, an...
Embodiment 3
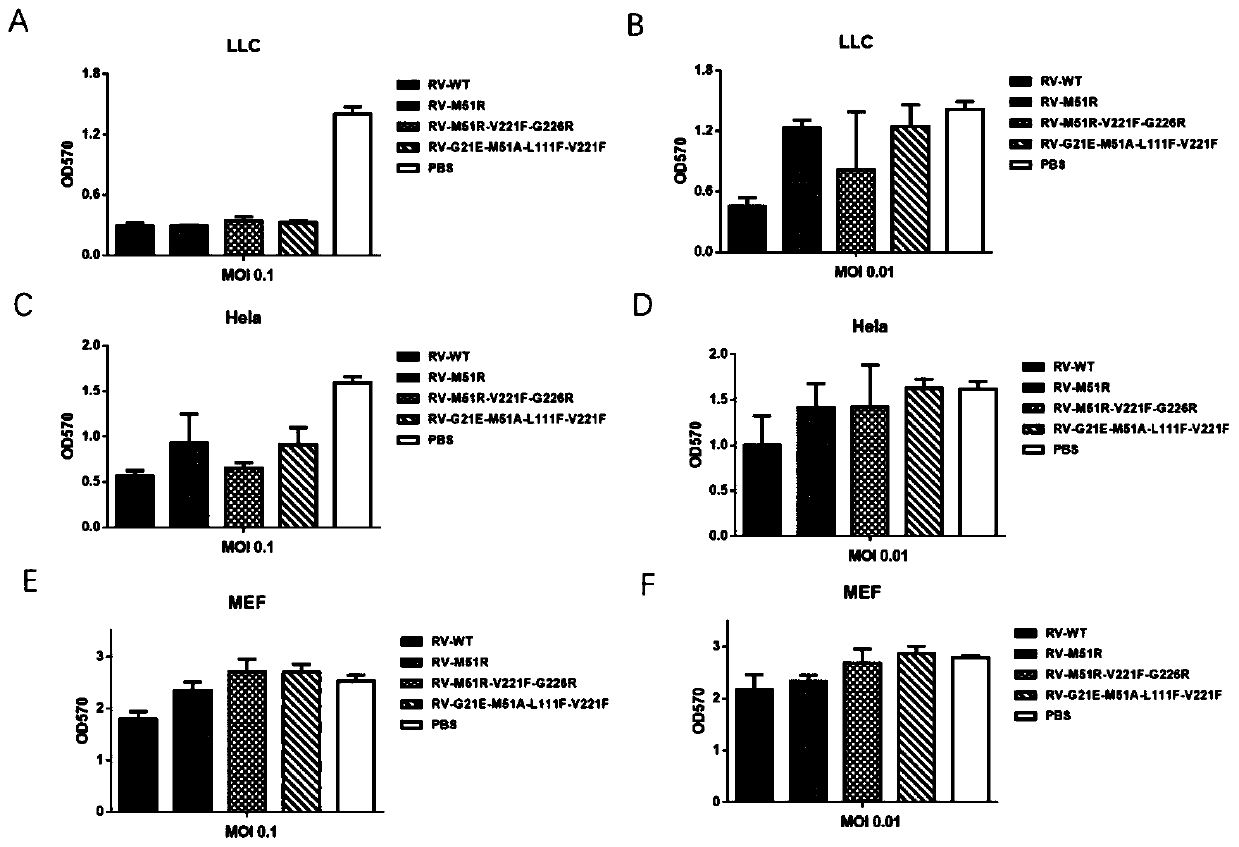
[0249] Example 3: Detection of the comparison of the in vitro killing of various tumor cells by RV-4Mut with different viral loads
[0250] By MTT detection method, the in vitro killing effect of RV-4Mut attenuated strains with different viral loads on different tumor cells was detected.
[0251] The specific steps of the above detection method are as follows:
[0252] 1. Add 100 μl of (LLC / Hela / MEF) cell suspension to each well of a 96-well culture plate to make the cell volume reach 1×10 4 pc / well, 37°C, 5% CO 2 Cultivate for 16h.
[0253] 2. Dilute the viruses RV-WT, RV-M51R, RV-M51R-V221F-G226R (RV-3Mut), RV-G21E-M51A-L111F-V221F (RV-4Mut) to an MOI (multiplicity of infection) of 0.001, respectively , 0.01, 0.1, 1.0, inoculate 4 wells for each dilution gradient, 100 μl per well, 37°C, 5% CO 2 Cultivate for 40h.
[0254] 3. Discard the supernatant in the 96-well culture plate, add fresh medium, and add MTT solution, 20 μL / well. 37°C, 5% CO 2 Cultivate for 4h.
[025...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com