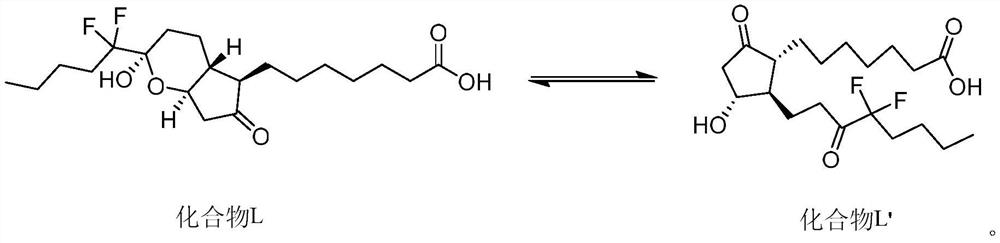
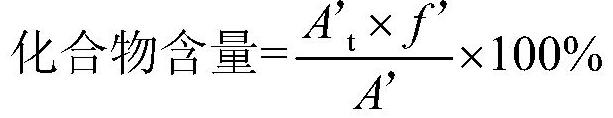A kind of analytical method for determination of lubiprostone test substance related substances
A lubiprostone and analysis method technology, applied in the field of drug analysis, can solve problems such as the instability of lubiprostone structure, and achieve the effect of ensuring controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
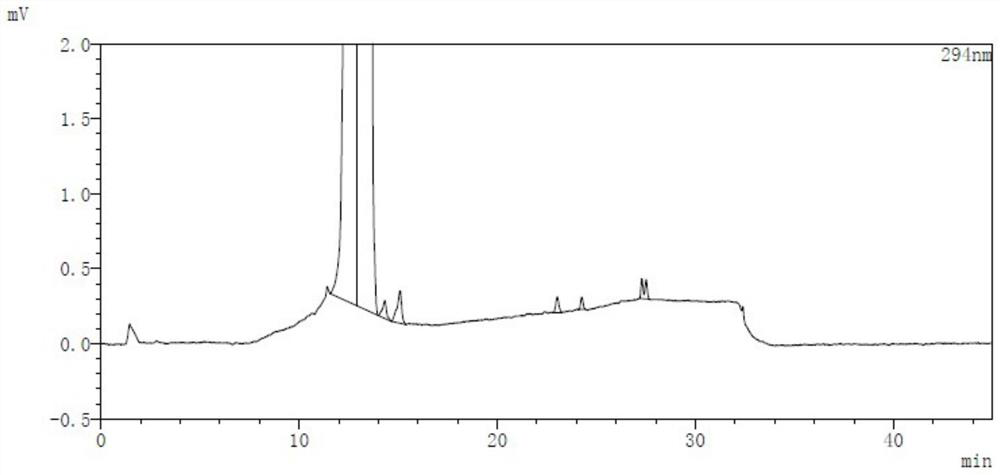
[0080] Embodiment 1 Determination method of related substances
[0081] Take an appropriate amount of lubiprostone test product, accurately weigh it, dissolve and dilute it with acetonitrile to make a solution containing about 5 mg per 1ml, as the test solution; take an appropriate amount of lubiprostone reference substance, accurately weigh it, and use Acetonitrile is dissolved and diluted to make a solution containing about 10 μg per 1ml, as a reference solution; another appropriate amount of lubiprostone, compound of formula I, compound of formula II, compound of formula III, compound of formula IV, compound of formula V reference substance is taken. , accurately weighed, dissolved in acetonitrile and diluted respectively to make a solution containing about 5 mg of lubiprostone per 1 ml, a solution containing about 5 μg of the compound of formula I per 1 ml, and a solution of about 5 μg of the compound of formula II per 1 ml, Each 1 ml of a solution containing about 5 μg of...
Embodiment 2
[0090] Embodiment 2 Reproducibility experiment of determination of related substances
[0091] Take an appropriate amount of the test sample, add acetonitrile to dissolve and dilute to make a solution containing about 5mg per 1ml, as the test sample solution, prepare six copies in parallel, according to the method described in Example 1, sample injection analysis, and calculate with the self-control method Each impurity and total impurity content were investigated to investigate the repeatability of this method. The measurement results are shown in the table below:
[0092] Repeat test results table
[0093] sample 1 2 3 4 5 6 Compound of formula III (%) 0.04 0.04 0.04 0.04 0.04 0.05 Compound of formula I (%) not detected not detected not detected not detected not detected not detected Compound of formula IV (%) not detected not detected not detected not detected not detected not detected Compound of formula II (...
Embodiment 3
[0095] Example 3 Intermediate Precision Experiment of Related Substances
[0096] According to the method described in Example 1, two analysts respectively measured each impurity and total impurity content on two different high-performance liquid chromatographs according to the method for determination of related substances. The intermediate precision results of the method for the determination of the substances are shown in the following table:
[0097] Table of intermediate precision results for related substances
[0098]
[0099] As can be seen from the above table, the results of the intermediate precision show that the content of the compound of formula III is 0.03% to 0.05%, the maximum single impurity content is: 0.03% to 0.04%, and the total impurity content fluctuates between 0.07% and 0.10%. The method has good intermediate precision.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com